Compound
| Pills | 1 table |
| active substance: | |
| nimesulide | 100 mg |
| excipients: lactose - 151.5 mg; croscarmellose sodium - 22 mg; colloidal silicon dioxide - 11 mg; corn starch - 37.6 mg; povidone - 8.5 mg; docusate sodium - 6.8 mg; polysorbate - 1 mg; hydrochloric acid*; purified water*; magnesium stearate - 1.6 mg (* removed during production) |
| Lozenges | 1 table |
| active substance: | |
| nimesulide | 100 mg |
| excipients: mannitol - 187.85 mg; sodium carboxymethyl starch - 15 mg; sodium methyl parahydroxybenzoate - 0.281 mg; sodium propyl parahydroxybenzoate - 0.021 mg; potassium sorbate - 0.346 mg; croscarmellose sodium - 3 mg; colloidal silicon dioxide - 5 mg; magnesium stearate - 5 mg; aspartame - 7 mg; orange flavor - 1.5 mg |
Pharmacodynamics
Nimulide is considered an NSAID from the sulfonanilide class. It has anti-inflammatory, analgesic and antipyretic effects.
Nimesulide is a NSAID, the mechanism of action of which is associated with selective inhibition of COX-2 and effects on a number of other factors - suppression of platelet activating factor, tumor necrosis factor alpha, suppression of proteinases and histamine. By selectively suppressing COX-2, it reduces the biosynthesis of PG at the site of inflammation, has a less pronounced inhibitory effect on COX-1 (less likely to cause side effects associated with inhibition of PG synthesis in healthy tissues).
Analogues and prices
Among foreign and Russian analogues of Nimulid there are:
Nimika. Manufacturer: Ipka (India). Price in pharmacies from 247 rubles. Nimesulide. Manufacturer: Teva 199 rub. Nise. Manufacturer: Dr. Reddy's (India). Price in pharmacies from 183 rubles. Nimesil. Manufacturer: Guidotti Laboratory S.p.A. (Germany). Price in pharmacies from 692 rubles.
Pharmacokinetics
Absorption when taken orally is high (food intake reduces the rate of absorption without affecting its degree). Communication with plasma proteins - 95%, with erythrocytes - 2%, with lipoproteins - 1%, with acidic alpha 1-glycoproteins - 1%. Changing the dosage does not affect the degree of binding. Cmax - 3.5–6.5 mg/l. Vd - 0.19–0.35 l/kg.
Penetrates into the tissues of the female genital organs, where after a single dose its concentration is about 40% of the concentration in plasma. Normally penetrates into the acidic environment of the inflammation site (40%), synovial fluid (43%). Easily penetrates histohematic barriers.
Metabolized in the liver by tissue monooxygenases. The main metabolite, 4-hydroxynimesulide (25%), has similar pharmacological activity, but due to a decrease in molecular size, it is able to quickly diffuse through the hydrophobic COX-2 channel to the active binding site of the methyl group. 4-Hydroxynimesulide is considered a water-soluble compound, the elimination of which does not require glutathione and phase II metabolic conjugation reactions (including sulfation, glucuronidation).
T1/2 of nimesulide is about 1.56–4.95 hours, 4-hydroxynimesulide is 2.89–4.78 hours. 4-Hydroxynimesulide is excreted by the kidneys (65%) and bile (35%), and undergoes enterohepatic recirculation.
In patients with renal failure (creatinine clearance 1.8–4.8 l/h or 30–80 ml/min), as well as in children and elderly people, the pharmacokinetic profile of nimesulide does not change significantly.
Nimulid
pharmachologic effect
Nimulide is an NSAID from the sulfonalilide class.
Has anti-inflammatory, analgesic and antipyretic effects. Nimesulide is a NSAID, the mechanism of action of which is associated with selective inhibition of cyclooxygenase-2 (COX-2) and effects on a number of other factors: suppression of platelet activating factor, tumor necrosis factor alpha, suppression of proteinases and histamine. By selectively suppressing COX-2, it reduces the biosynthesis of prostaglandins at the site of inflammation and has a less pronounced inhibitory effect on COX-1 (less likely to cause side effects associated with inhibition of prostaglandin synthesis in healthy tissues).
Pharmacokinetics
Absorption when taken orally is high (food intake reduces the rate of absorption without affecting its degree). Binding to plasma proteins - 95%, to erythrocytes - 2%, to lipoproteins - 1%, to acidic alpha1-glycoproteins - 1%. Changing the dose does not affect the degree of binding. Cmax - 3.5-6.5 mg/l. Vd - 0.19-0.35 l/kg. Penetrates into the tissues of the female genital organs, where after a single dose its concentration is about 40% of the concentration in plasma. Penetrates well into the acidic environment of the inflammation site (40%) and synovial fluid (43%). Easily penetrates histohematic barriers.
Metabolized in the liver by tissue monooxygenases. The main metabolite, 4-hydroxynimesulide (25%), has similar pharmacological activity, but due to a decrease in molecular size, it is able to quickly diffuse through the hydrophobic COX-2 channel to the active binding site of the methyl group. 4-hydroxynimesulide is a water-soluble compound, the elimination of which does not require glutathione and conjugation reactions of phase II metabolism (sulfation, glucuronidation, etc.).
T1/2 of nimesulide is about 1.56-4.95 hours, 4-hydroxynimesulide is 2.89-4.78 hours. 4-hydroxynimesulide is excreted by the kidneys (65%) and bile (35%), and undergoes enterohepatic recirculation.
In patients with renal failure (creatinine clearance 1.8-4.8 l/h or 30-80 ml/min), as well as in children and the elderly, the pharmacokinetic profile of nimesulide does not change significantly.
Indications
- rheumatoid arthritis;
- articular syndrome during exacerbation of gout;
- psoriatic arthritis;
— ankylosing spondylitis, osteochondrosis with radicular syndrome;
- osteoarthritis;
— myalgia of rheumatic and non-rheumatic origin;
- inflammation of ligaments, tendons, bursitis, including post-traumatic inflammation of soft tissues;
— pain syndrome of various origins (including in the postoperative period, with injuries, algodismenorrhea, toothache, headache, arthralgia, lumbar ischialgia);
- symptomatic therapy to reduce pain and inflammation at the time of use, does not affect the progression of the disease.
Dosage regimen
Adults and children over 12 years of age
(body weight more than 40 kg)
are prescribed 1 tablet orally. 2 times/day.
The tablets are taken after meals with sufficient water. The maximum daily dose is 5 mg/kg.
Patients with chronic renal failure
a reduction in the daily dose to 100 mg is required.
Course of treatment: as prescribed by the doctor.
Side effect
From the digestive system:
often - diarrhea, nausea, vomiting; infrequently - constipation, flatulence, gastritis; very rarely - abdominal pain, stomatitis, tarry stools, gastrointestinal bleeding, ulcer and/or perforation of the stomach or duodenum.
From the side of the central nervous system:
infrequently - dizziness; rarely - a feeling of fear, nervousness, nightmares; very rarely - headache, drowsiness, encephalopathy (Reye's syndrome).
From the respiratory system:
infrequently - shortness of breath; very rarely - bronchospasm; Possible exacerbation of bronchial asthma.
From the cardiovascular system:
infrequently - arterial hypertension; rarely - tachycardia, hemorrhages, hot flashes.
Sense organs:
rarely - blurred vision.
From the skin and mucous membranes:
uncommon - itching, rash, increased sweating; rarely - erythema, dermatitis.
From the liver and biliary system:
often - increased activity of liver transaminases; very rarely - hepatitis, fulminant hepatitis, jaundice, cholestasis.
From the urinary system:
infrequently - swelling; rarely - dysuria, hematuria, urinary retention, hyperkalemia; very rarely - renal failure, oliguria, interstitial nephritis.
From the hematopoietic system:
rarely - anemia, eosinophilia; very rarely - thrombocytopenia, pancytopenia, purpura, prolonged bleeding time.
Allergic reactions:
rarely - hypersensitivity reactions; very rarely - anaphylachtoid reactions, urticaria, angioedema, facial swelling, exudative erythema multiforme, incl. Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell's syndrome).
General reactions:
rarely - general weakness; very rarely - hypothermia.
If you experience other side effects not mentioned above or if your health worsens, please contact your doctor immediately.
Contraindications
- erosive and ulcerative changes in the mucous membrane of the stomach and duodenum, incl. inflammatory bowel diseases in the acute phase;
- active gastrointestinal bleeding;
- severe impairment of liver and/or kidney function, severe renal failure (creatinine clearance less than 30 ml/min), progressive kidney disease;
- severe heart failure;
- severe blood clotting disorders;
- confirmed hyperkalemia;
— the period after coronary artery bypass grafting;
- anamnestic data on an attack of bronchial obstruction, rhinitis, urticaria after taking acetylsalicylic acid or other NSAIDs (complete or incomplete acetylsalicylic acid intolerance syndrome - rhinosinusitis, urticaria, nasal polyps, asthma);
- severe liver failure or active liver disease;
- pregnancy;
- lactation period;
- children under 12 years of age;
- hypersensitivity to nimesulide and the components of the drug.
Carefully :
coronary heart disease, cerebrovascular disease, congestive heart failure, dyslipidemia/hyperlipidemia, diabetes mellitus, peripheral arterial disease, smoking, creatinine clearance less than 60 ml/min, history of gastrointestinal ulceration, presence of Helicobacter pylori infection, older age, long-term use NSAIDs, frequent alcohol consumption, severe somatic diseases, concomitant therapy with the following drugs:
- anticoagulants (for example, warfarin);
- antiplatelet agents (for example, acetylsalicylic acid, clopidogrel);
- oral corticosteroids (for example, prednisolone);
- selective serotonin reuptake inhibitors (for example, citalopram, fluoxetine, paroxetine, sertraline).
Pregnancy and lactation
The drug is contraindicated during pregnancy and lactation.
special instructions
Nimulide should be used with caution in patients with a history of bleeding, patients with upper gastrointestinal diseases, or patients receiving anticoagulants.
Since Nimulide is partially excreted by the kidneys, its dosage for patients with impaired renal function should be reduced, depending on creatinine clearance.
Given reports of visual disturbances in patients taking other NSAIDs, treatment should be stopped immediately if any visual disturbance occurs and the patient should be examined by an ophthalmologist.
The drug can cause fluid retention in tissues, so patients with high blood pressure and cardiac problems should use Nimulid with extreme caution.
To reduce the risk of developing adverse events from the gastrointestinal tract, the minimum effective dose should be used for the shortest possible short course.
Impact on the ability to drive vehicles and operate machinery
Patients who experience severe side effects: dizziness, drowsiness, blurred vision, must be careful when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Overdose
Symptoms:
apathy, drowsiness, nausea, vomiting. Gastrointestinal bleeding, arterial hypertension, acute renal failure, and respiratory depression may occur.
Treatment:
Symptomatic treatment of the patient is required; there is no specific antidote. If an overdose occurs within the last 4 hours, it is necessary to induce vomiting, take activated carbon (60-100 g per adult), and osmotic laxatives. Forced diuresis and hemodialysis are ineffective due to the high binding of the drug to proteins.
Drug interactions
The effect of medications that reduce blood clotting is enhanced when used simultaneously with nimesulide.
Nimesulide may reduce the effect of furosemide. Reduces the therapeutic effect of antihypertensive drugs. Nimesulide increases the occurrence of side effects while taking methotrexate.
Plasma lithium levels increase when lithium and nimesulide are taken simultaneously.
Nimesulide may enhance the nephrotoxic effect of cyclosporine on the kidneys. Use with glucocorticosteroids and serotonin reuptake inhibitors increases the risk of developing gastrointestinal bleeding.
Conditions for dispensing from pharmacies
The drug is available with a prescription.
Storage conditions and periods
Store the drug at a temperature of 15°-25°C, in a dry place protected from light. Keep out of the reach of children.
Shelf life: 5 years. Do not use a drug that has expired.
Contraindications
Pills
hypersensitivity to nimesulide and drug components;
erosive and ulcerative changes in the mucous membrane of the stomach and duodenum;
inflammatory bowel diseases in the acute phase;
active gastrointestinal bleeding;
severe impairment of liver and/or kidney function, severe renal failure (Cl creatinine <30 ml/min);
progressive kidney disease;
severe liver failure or active liver disease;
severe heart failure;
severe blood clotting disorders;
confirmed hyperkalemia;
period after coronary artery bypass surgery;
anamnestic data on an attack of bronchial obstruction, rhinitis, urticaria after taking acetylsalicylic acid or other NSAIDs (complete or incomplete acetylsalicylic acid intolerance syndrome - rhinosinusitis, urticaria, nasal polyps, asthma);
pregnancy and breastfeeding;
children's age up to 12 years.
Lozenges
hypersensitivity to the active substance or auxiliary components;
complete or incomplete combination of bronchial asthma, recurrent nasal polyposis or paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including a history);
erosive and ulcerative changes in the mucous membrane of the stomach or duodenum, active gastrointestinal bleeding; cerebrovascular or other bleeding;
inflammatory bowel diseases (ulcerative colitis, Crohn's disease) in the acute phase;
hemophilia and other bleeding disorders;
decompensated heart failure;
liver failure or any active liver disease;
severe renal failure (creatinine Cl <30 ml/min);
progressive kidney disease;
confirmed hyperkalemia;
period after coronary artery bypass surgery;
anamnestic data on the development of hepatotoxic reactions when using nimesulide preparations;
concomitant use of potentially hepatotoxic substances;
alcoholism, drug addiction;
pregnancy, breastfeeding period;
children's age up to 12 years.
With caution (tablets, lozenges): coronary heart disease, cerebrovascular diseases, congestive heart failure, dyslipidemia/hyperlipidemia, diabetes mellitus, peripheral arterial disease, smoking, creatinine Cl <60 ml/min.
Anamnestic data on the development of ulcerative lesions of the gastrointestinal tract, the presence of Helicobacter pylori infection, old age, long-term use of NSAIDs, frequent alcohol consumption, severe somatic diseases, concomitant therapy with the following medications - anticoagulants (in particular warfarin), antiplatelet agents (in particular acetylsalicylic acid, clopidogrel), oral corticosteroids (in particular prednisolone), SSRIs (in particular citalopram, fluoxetine, paroxetine, sertraline).
Pharmacological group
The main active substance of the drug Nimulid is nimesulide, which, according to its chemical derivative, belongs to the class of sulfonamides. The drug inhibits the production of the main mediators of the inflammatory response and prostaglandins from sugar acid. This reaction is achieved by inhibiting the enzyme cyclooxygenase. By reducing the concentration of prostaglandins in the area of the inflammatory process, swelling and pain are eliminated.
Unlike other non-steroidal anti-inflammatory drugs, Nimesulide acts selectively, inhibiting almost cyclooxygenases, with virtually no effect on the activity of tsog-1. The drug does not have a negative effect on the level of prostaglandins located on the gastric mucosa.
Immediately after oral administration of the drug, Nimulid and its active substance are fully and quickly absorbed into the systemic circulation from the intestinal lumen. The composition is well and quickly distributed in tissues with high accumulation in an acidic environment. Metabolism of the drug component is ensured in liver cells. In the process, inactive decay products are formed, which are excreted from the human body with urine and bile. The half-life is up to 5 hours.
| Category ICD-10 | Synonyms of diseases according to ICD-10 |
| M06.9 Rheumatoid arthritis, unspecified | Rheumatoid arthritis |
| Pain syndrome in rheumatic diseases | |
| Rheumatoid arthritis pain | |
| Inflammation in rheumatoid arthritis | |
| Degenerative forms of rheumatoid arthritis | |
| Pediatric rheumatoid arthritis | |
| Exacerbation of rheumatoid arthritis | |
| Acute rheumatism | |
| Acute rheumatoid arthritis | |
| Acute articular rheumatism | |
| Rheumatoid arthritis | |
| Rheumatic arthritis | |
| Rheumatoid arthritis | |
| Rheumatic arthritis | |
| Rheumatoid arthritis | |
| Rheumatoid arthritis | |
| Active rheumatoid arthritis | |
| Rheumatoid periarthritis | |
| Rheumatoid polyarthritis | |
| M07.3 Other psoriatic arthropathies (L40.5) | Psoriatic arthritis |
| Generalized form of psoriatic arthritis | |
| Psoriatic arthritis | |
| M10.9 Gout, unspecified | Gouty arthritis |
| Acute gout | |
| Acute gouty arthritis | |
| Acute attack of gout | |
| Gouty arthritis | |
| Salt diathesis | |
| Articular syndrome during exacerbation of gout | |
| Joint syndrome with gout | |
| Uraturia | |
| Chronic gouty arthritis | |
| M19.9 Arthrosis, unspecified | Arthrosis |
| Arthrosis deforming | |
| Arthrosis of large joints | |
| Pain syndrome in osteoarthritis | |
| Pain syndrome in osteoarthritis | |
| Pain syndrome in acute inflammatory diseases of the musculoskeletal system | |
| Pain syndrome in chronic inflammatory diseases of the musculoskeletal system | |
| Deforming arthrosis | |
| Deforming osteoarthritis | |
| Deforming osteoarthritis of joints | |
| Changes in the hand with osteoarthritis | |
| Osteoarthritis | |
| Osteoarthritis in the acute stage | |
| Osteoarthritis of large joints | |
| Acute pain syndrome in osteoarthritis | |
| Post-traumatic osteoarthritis | |
| Rheumatic osteoarthritis | |
| Spondylarthrosis | |
| Chronic osteoarthritis | |
| M42 Osteochondrosis of the spine | Pain due to spinal osteochondrosis |
| Radicular syndrome in osteochondrosis | |
| Intervertebral osteochondrosis | |
| Osteochondrosis | |
| Osteocondritis of the spine | |
| Osteochondrosis with radicular syndrome | |
| Cervical osteochondrosis | |
| M45 Ankylosing spondylitis | Ankylosing spondylitis |
| Ankylosing spondylitis | |
| Ankylosing spondylitis | |
| Pain syndrome in acute inflammatory diseases of the musculoskeletal system | |
| Pain syndrome in chronic inflammatory diseases of the musculoskeletal system | |
| Diseases of the spinal column | |
| Ankylosing spondylitis | |
| Ankylosing spondylitis-Marie-Strumpell disease | |
| Marie-Strumpel's disease | |
| Rheumatic spondylitis | |
| Ankylosing spondyloarthritis | |
| M54.1 Radiculopathy | Pain syndrome with radiculitis |
| Diseases of the spinal column | |
| Acute radicular radiculopathy | |
| Acute radiculitis | |
| Subacute radiculitis | |
| Radiculitis | |
| Radiculitis | |
| Radiculitis with radicular syndrome | |
| Radiculopathy | |
| Chronic radiculitis | |
| M54.3 Sciatica | Sciatica |
| Neuralgia of the sciatic nerve | |
| Sciatic nerve neuritis | |
| M54.4 Lumbago with sciatica | Pain in the lumbosacral spine |
| Lumbago | |
| Lumbar syndrome | |
| Sciatica | |
| M54.5 Pain in the lower back | Painful conditions of the spinal column |
| Lower back pain | |
| Lower back pain | |
| Lower back pain | |
| Lower back pain | |
| Lumbar pain | |
| Lumbodynia | |
| Low back pain syndrome | |
| M67.9 Lesion of synovium and tendon, unspecified | Ligament inflammation |
| Tendon inflammation | |
| Tendon inflammation due to injury | |
| Tendon inflammation | |
| M71.9 Bursopathy, unspecified | Alberta disease |
| Bursitis | |
| Acute bursitis | |
| M79.1 Myalgia | Pain syndrome in muscular and joint diseases |
| Pain syndrome in chronic inflammatory diseases of the musculoskeletal system | |
| Pain in the muscles | |
| Muscle soreness | |
| Muscle soreness during heavy physical activity | |
| Painful conditions of the musculoskeletal system | |
| Pain in the musculoskeletal system | |
| Muscle pain | |
| Pain at rest | |
| Muscle pain | |
| Muscle pain | |
| Musculoskeletal pain | |
| Myalgia | |
| Myofascial pain syndromes | |
| Muscle pain | |
| Muscle pain at rest | |
| Muscle pain | |
| Muscle pain of non-rheumatic origin | |
| Muscle pain of rheumatic origin | |
| Acute muscle pain | |
| Rheumatic pain | |
| Rheumatic pains | |
| Myofascial syndrome | |
| Fibromyalgia | |
| T14.3 Dislocation, sprain and damage to the capsular-ligamentous apparatus of a joint of an unspecified area of the body | Painful muscle strains |
| Pain and inflammation when stretched | |
| Reduction of dislocation | |
| Degenerative changes in the ligamentous apparatus | |
| Swelling due to sprains and bruises | |
| Swelling after interventions for dislocations | |
| Damage and rupture of ligaments | |
| Damage to the musculo-ligamentous apparatus | |
| Ligament damage | |
| Joint damage | |
| Habitual sprains and tears | |
| Ligament rupture | |
| Ligament tears | |
| Tendon ruptures | |
| Muscle tendon ruptures | |
| Joint injuries | |
| Stretching | |
| Crick | |
| Muscle strain | |
| Sprain | |
| Ligament sprain | |
| Tendon sprain | |
| Sprains | |
| Muscle strains | |
| Sprains | |
| Ligament sprains | |
| Tendon sprains | |
| Musculo-ligamentous injury | |
| Joint injuries | |
| Injuries to capsuloarticular tissues | |
| Injuries of the osteoarticular system | |
| Ligament injuries | |
| Joint injuries | |
| T14.9 Injury, unspecified | Pain syndrome after injuries |
| Pain syndrome due to injuries | |
| Pain syndrome during injuries and after surgery | |
| Pain from injuries | |
| Traumatic pain | |
| Joint pain due to injury | |
| Postoperative and post-traumatic pain | |
| Pain from injuries | |
| Pain of traumatic origin | |
| Severe pain syndrome of traumatic origin | |
| Deep tissue damage | |
| Deep scratches on the body | |
| Closed injury | |
| Minor domestic injuries | |
| Minor skin damage | |
| Violations of the integrity of soft tissues | |
| Uncomplicated injuries | |
| Extensive traumatic injury | |
| Acute pain syndrome of traumatic origin | |
| Swelling due to injuries | |
| Previous sports injuries | |
| Post-traumatic pain | |
| Soft tissue injuries | |
| Joint injuries | |
| Sports injuries | |
| Injury | |
| Traumatic pain | |
| Traumatic pain | |
| Traumatic infiltration | |
| Sports injuries | |
| Z100* CLASS XXII Surgical practice | Abdominal surgery |
| Adenomectomy | |
| Amputation | |
| Angioplasty of coronary arteries | |
| Carotid angioplasty | |
| Antiseptic treatment of skin for wounds | |
| Antiseptic hand treatment | |
| Appendectomy | |
| Atherectomy | |
| Balloon coronary angioplasty | |
| Vaginal hysterectomy | |
| Corona bypass | |
| Interventions on the vagina and cervix | |
| Bladder interventions | |
| Intervention in the oral cavity | |
| Restorative and reconstructive operations | |
| Hand hygiene of medical personnel | |
| Gynecological surgery | |
| Gynecological interventions | |
| Gynecological surgeries | |
| Hypovolemic shock during surgery | |
| Disinfection of purulent wounds | |
| Disinfection of wound edges | |
| Diagnostic interventions | |
| Diagnostic procedures | |
| Diathermocoagulation of the cervix | |
| Long surgical operations | |
| Replacing fistula catheters | |
| Infection during orthopedic surgery | |
| Artificial heart valve | |
| Cystectomy | |
| Short-term outpatient surgery | |
| Short-term operations | |
| Short-term surgical procedures | |
| Cricothyroidotomy | |
| Blood loss during surgery | |
| Bleeding during surgery and in the postoperative period | |
| Culdocentesis | |
| Laser coagulation | |
| Laser coagulation | |
| Laser coagulation of the retina | |
| Laparoscopy | |
| Laparoscopy in gynecology | |
| CSF fistula | |
| Minor gynecological operations | |
| Minor surgical interventions | |
| Mastectomy and subsequent plastic surgery | |
| Mediastinotomy | |
| Microsurgical operations on the ear | |
| Mucogingival surgeries | |
| Stitching | |
| Minor surgeries | |
| Neurosurgical operation | |
| Immobilization of the eyeball in ophthalmic surgery | |
| Orchiectomy | |
| Complications after tooth extraction | |
| Pancreatectomy | |
| Pericardectomy | |
| Rehabilitation period after surgery | |
| The period of convalescence after surgical interventions | |
| Percutaneous transluminal coronary angioplasty | |
| Pleural thoracentesis | |
| Pneumonia postoperative and post-traumatic | |
| Preparing for surgical procedures | |
| Preparing for surgery | |
| Preparing the surgeon's hands before surgery | |
| Preparing the colon for surgery | |
| Postoperative aspiration pneumonia during neurosurgical and thoracic operations | |
| Postoperative nausea | |
| Postoperative bleeding | |
| Postoperative granuloma | |
| Postoperative shock | |
| Early postoperative period | |
| Myocardial revascularization | |
| Resection of the apex of the tooth root | |
| Gastric resection | |
| Bowel resection | |
| Resection of the uterus | |
| Liver resection | |
| Small bowel resection | |
| Resection of part of the stomach | |
| Reocclusion of the operated vessel | |
| Bonding tissue during surgery | |
| Removing stitches | |
| Condition after eye surgery | |
| Condition after surgery | |
| Condition after surgical interventions in the nasal cavity | |
| Condition after gastrectomy | |
| Condition after resection of the small intestine | |
| Condition after tonsillectomy | |
| Condition after removal of the duodenum | |
| Condition after phlebectomy | |
| Vascular surgery | |
| Splenectomy | |
| Sterilization of surgical instruments | |
| Sterilization of surgical instruments | |
| Sternotomy | |
| Dental operations | |
| Dental intervention on periodontal tissues | |
| Strumectomy | |
| Tonsillectomy | |
| Thoracic surgery | |
| Thoracic operations | |
| Total gastrectomy | |
| Transdermal intravascular coronary angioplasty | |
| Transurethral resection | |
| Turbinectomy | |
| Removal of a tooth | |
| Cataract removal | |
| Cyst removal | |
| Tonsil removal | |
| Removal of fibroids | |
| Removal of mobile baby teeth | |
| Removal of polyps | |
| Removing a broken tooth | |
| Removal of the uterine body | |
| Removing stitches | |
| Urethrotomy | |
| CSF duct fistula | |
| Frontoethmoidohaymorotomy | |
| Surgical infection | |
| Surgical treatment of chronic limb ulcers | |
| Surgery | |
| Surgery in the anal area | |
| Colon surgery | |
| Surgical practice | |
| Surgical procedure | |
| Surgical interventions | |
| Surgical interventions on the gastrointestinal tract | |
| Surgical interventions on the urinary tract | |
| Surgical interventions on the urinary system | |
| Surgical interventions on the genitourinary system | |
| Heart surgery | |
| Surgical procedures | |
| Surgical operations | |
| Vein surgery | |
| Surgical intervention | |
| Vascular surgery | |
| Surgical treatment of thrombosis | |
| Surgery | |
| Cholecystectomy | |
| Partial gastrectomy | |
| Transperitoneal hysterectomy | |
| Percutaneous transluminal coronary angioplasty | |
| Percutaneous transluminal angioplasty | |
| Coronary artery bypass surgery | |
| Tooth extirpation | |
| Extirpation of baby teeth | |
| Pulp extirpation | |
| Extracorporeal circulation | |
| Tooth extraction | |
| Tooth extraction | |
| Cataract extraction | |
| Electrocoagulation | |
| Endourological interventions | |
| Episiotomy | |
| Ethmoidotomy |
Side effects
Frequency is classified depending on occurrence - very common (>10); often (<10–<100); uncommon (<100–<1000); rare (<1000–<10000); very rare (<10,000).
From the gastrointestinal tract: often - diarrhea, nausea, vomiting; infrequently - constipation, flatulence, gastritis; very rarely - abdominal pain, stomatitis, tarry stools, gastrointestinal bleeding, ulcer and/or perforation of the stomach or duodenum.
From the side of the central nervous system: infrequently - dizziness; rarely - a feeling of fear, nervousness, nightmares; very rarely - headache, drowsiness, encephalopathy (Reye's syndrome).
From the respiratory system: infrequently - shortness of breath; very rarely - bronchospasm; likely exacerbation of bronchial asthma.
From the cardiovascular system: infrequently - arterial hypertension; rarely - tachycardia, hemorrhages, hot flashes.
From the senses: rarely - blurred vision.
Skin and mucous membranes: infrequently - itching, rash, increased sweating; rarely - erythema, dermatitis.
Liver and biliary system: often - increased activity of liver transaminases; very rarely - hepatitis, fulminant hepatitis, jaundice, cholestasis.
Kidneys and urinary system: infrequently - edema; rarely - dysuria, hematuria, urinary retention, hyperkalemia; very rarely - renal failure, oliguria, interstitial nephritis.
From the hematopoietic organs: rarely - anemia, eosinophilia; very rarely - thrombocytopenia, pancytopenia, purpura, prolonged bleeding time.
Allergic reactions: rarely - hypersensitivity reactions; very rarely - anaphylactoid reactions, urticaria, angioedema, facial swelling, exudative erythema multiforme, incl. Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell's syndrome).
General reactions: rarely - general weakness; very rarely - hypothermia.
If other side effects not mentioned above occur or if you feel unwell, you should consult a doctor immediately.
Interaction
The effect of medications that reduce blood clotting is enhanced when used simultaneously with nimesulide.
Nimesulide may reduce the effects of furosemide. Reduces the therapeutic effect of antihypertensive drugs. Nimesulide may increase the potential for side effects while taking methotrexate.
Plasma lithium levels increase when lithium and nimesulide are taken simultaneously.
Due to the high degree of binding of nimesulide to plasma proteins, patients who are simultaneously treated with hydantoin and sulfonamides should be under medical supervision, undergoing studies at short intervals.
Nimesulide may increase the effects of cyclosporine on the kidneys.
Use with GCS and serotonin reuptake inhibitors increases the risk of bleeding from the gastrointestinal tract.
Application in various countries
India
Several reports have been made of adverse drug reactions in India.[7][8][9][10] On 12 February 2011, the Indian Express reported that the Union Ministry of Health and Family Welfare had finally decided to suspend the pediatric use of the painkiller, nimesulide suspension.[11] Since March 10, 2011, Nimesulide preparations are not indicated for human use in children under 12 years of age.[12]
On 13 September 2011, the Madras High Court lifted the stay on the manufacture and sale of the pediatric drugs nimesulide and phenylpropanolamine (PPA).[13]
Europe
On 21 September 2007, the EMA issued a press release about the review of the liver-related safety of nimesulide. The EMA concluded that the benefits of these medicines outweigh their risks, but the duration of use must be limited to ensure that the risk of developing liver disease is minimized. Therefore, the EMA limits the use of systemic preparations (tablets, solutions, suppositories) of nimesulide to 15 days.[14]
Ireland
The Irish Medicines Board (IMB) has decided to suspend the marketing of Nimesulide from the Irish market and refer it to the EU Committee for Medicinal Products for Human Use (CHMP) for a review of its benefit/risk profile. This decision follows the report of six cases potentially associated with liver failure by the IMB National Liver Transplant Unit, St. Vincent's Hospital. These cases occurred between 1999 and 2006.[15]
Bribes
In May 2008, the leading Italian daily Corriere della Sera and other media reported that a senior official of the Italian medicines agency AIFA was filmed by police accepting bribes from pharmaceutical company employees.[16][17] The money was allegedly paid to ensure less scrutiny control by responsible authorities. The investigation began in 2005 after suspicions that some AIFA drug tests were tampered with. Eight arrests were made. Nimesulide can be purchased with a prescription from a doctor, which remains in the pharmacy, nominally ensuring strict control over the sale of the drug.
The original manufacturer of Nimesulide is Helsinn Healthcare SA (Switzerland), which acquired the rights to this drug in 1976. After patent protection ended, a number of other companies began producing and marketing Nimesulide.
Directions for use and doses
Pills
Inside. Adults and children over 12 years old (body weight >40 kg) - 1 tablet. 2 times a day. The tablets are taken after meals with sufficient liquid. The highest daily dosage is 5 mg/kg.
Patients with chronic renal failure require a reduction in the daily dosage to 100 mg.
The course of treatment is as prescribed by the doctor.
Lozenges
The minimum effective dose should be used in the shortest possible short course.
Adults and children over 12 years of age (body weight >40 kg) - often at a dose of 100 mg (1 tablet) 2 times a day at the end or after meals. The tablet should be placed on the tongue, where it immediately begins to dissolve. Swallow the saliva in which the tablet has dissolved.
Overdose
Symptoms: apathy, drowsiness, nausea, vomiting. Gastrointestinal bleeding, arterial hypertension, acute renal failure, and respiratory depression may occur.
Treatment: The patient requires symptomatic treatment and supportive care. There is no specific antidote. If an overdose has occurred within the last 4 hours, it is necessary to induce vomiting, take activated carbon (60–100 g per adult), and osmotic laxatives. Forced diuresis and hemodialysis are ineffective due to the high binding of the drug to proteins.
special instructions
Nimulide should be used with caution in patients with a history of bleeding, patients with upper gastrointestinal disease, or patients receiving anticoagulants. Since Nimulide is partially excreted by the kidneys, its dosage for patients with impaired renal function should be reduced depending on creatinine Cl levels.
Given reports of visual disturbances in patients taking other NSAIDs, treatment should be promptly discontinued if any visual disturbance occurs and the patient should be examined by an ophthalmologist.
The drug can cause fluid retention in tissues, so patients with high blood pressure and cardiac problems should use Nimulid with extreme caution.
To reduce the risk of developing adverse events from the gastrointestinal tract, the minimum effective dose should be used in the shortest possible short course.
Impact on the ability to drive a car or perform work that requires increased speed of physical and mental reactions. Patients who experience side effects such as dizziness, drowsiness, blurred vision should be careful when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Indications for use for children
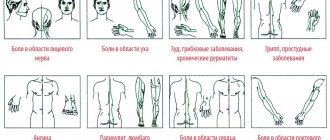
Nimulid is prescribed to children for fever or pain symptoms of various origins:
- upper respiratory tract diseases;
- toothache or post-operative pain;
- inflammatory processes of the ear, nose;
- inflammation of ligaments and muscles.
The most common indications for prescribing Nimulid to children are fever, pain due to teeth growth, ARVI, influenza or colds.
The medicine is available in various forms: syrup, gel, tablets and suspension. Children are prescribed Nimulid in the form of a suspension.
Please note: suspension and syrup are different forms of product release. The syrup is a completely soluble drug, and the suspension contains small granules that settle to the bottom. The suspension must be shaken before use.