The role of phosphorus in the human body
In addition to the above-mentioned functions of phosphorus, it also regulates the formation of certain hormonal elements, and also takes part in the metabolism of fats, carbohydrates and proteins. In addition, it helps to constantly maintain the acidic environment of the body. Other vital functions of phosphorus include:
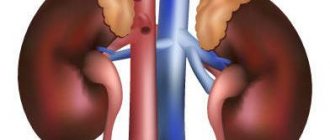
- improved kidney function;
- regulation of metabolism;
- formation of vital energy for the functioning of the heart and the whole body;
- participation in cell division;
- strengthening teeth, gums and bones;
- controlling the functioning of the nervous system.
Content
- 1. History
- 2 Origin of the name
- 3 Receipt
- 4 Physical properties 4.1 White phosphorus
- 4.2 Yellow phosphorus
- 4.3 Red phosphorus
- 4.4 Black phosphorus
- 4.5 Metallic phosphorus
- 5.1 Interaction with simple substances
- 7.1 Elemental phosphorus
- 8.1 Toxicology of elemental phosphorus
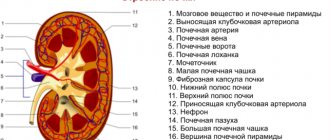
How phosphorus is absorbed in the human body
This macroelement is absorbed in the stomach and small intestine with the assistance of elements such as potassium, magnesium, iron, and vitamins, and, can also be such activators of its absorption. In addition, in order for the body to better absorb phosphorus, the presence of certain enzymes and necessarily proteins is necessary. Important for its absorption is its combination with calcium, which almost doubles this process. As for those substances that, on the contrary, inhibit the process of phosphorus absorption, they are aluminum, estrogens, corticosteroids, thyroxine and androgens.
White death: why phosphorus is prohibited by the laws of war
The human rights organization Human Rights Watch accused the Syrian Democratic Forces of using white phosphorus during the battle for Raqqa. According to international observers, the use of incendiary weapons led to the death of dozens of civilians in the capital of the Islamic State (a terrorist organization banned in Russia). The rebels received phosphorus shells from the United States. The Pentagon does not deny this fact, but department representatives insist: incendiary ammunition is used in Raqqa solely for camouflage and signaling. MIR 24 figured out why white phosphorus is so dangerous and why it is prohibited by international agreements.
Phosphorus ammunition began to be used in the 19th century. Irish terrorists who fought for the independence of their country were very fond of the dangerous substance. At the same time, phosphorus was banned by the St. Petersburg Declaration “On the abolition of the use of explosive and incendiary bullets.” The agreement was broken during the First World War, when phosphorus began to be used as a weapon of mass destruction. Both the Entente countries and the Triple Alliance resorted to barbaric methods of warfare.
During the interbellum period, the leading powers abandoned the production of incendiary shells. However, in the late 30s, the aggressive policies of Nazi Germany forced the Allies to return to the development of chemical weapons. During the war, phosphorus was used not only by the army, but also by ordinary partisans, who disguised the dangerous composition as ordinary soap.
In 1977, an additional protocol to the Geneva Convention was adopted, which finally banned the use of phosphorus in cases where its victims could be civilians. The US and Israel refused to sign the document. These states are often accused of violating the laws of war and using prohibited weapons.
Use of AN-M47-Phosphorbombe 1966 in the Vietnam War Photo: USAF, Wikipedia
In addition, experts point to the psychological factor of using phosphorus. The sight of a person covered with deep burns and who is difficult to help with anything is shocking to anyone. But an even greater threat is the way phosphorus can burn down entire residential areas. It is not easy to extinguish such a flame - water cannot completely block access to oxygen, which ignites the substance.
Unfortunately, no conventions have saved humanity from such a terrible weapon as white phosphorus.
One of the tragic pages of recent times is Operation Anfal, which was carried out by the Iraqi military under the leadership of Saddam Hussein. To genocide the Kurdish population, the army repeatedly used a mixture of phosphorus, mustard gas and other toxic substances. Subsequently, the mass murder of civilians with chemical weapons became one of the formal reasons for the American invasion of Iraq and the execution of the dictator.
Not only the Middle East, but also the very heart of Europe - Yugoslavia - suffered from white phosphorus. During the siege of Sarajevo, the Bosnian Serbs repeatedly used incendiary munitions, which injured many civilians. Phosphorus charges in Sarajevo also destroyed the Institute of Oriental Studies, and most of the rare archive was irretrievably lost.
But the infamous siege of Iraqi Fallujah caused an even greater resonance. During the assault on the city, the US military repeatedly used white phosphorus in densely populated areas. Pentagon representatives initially denied the use of prohibited weapons, but soon enough the press secretary of the military department, Barry Venable, had to make an official statement. He admitted that the US Army used prohibited weapons, but only against the enemy. The military department then also recalled that Washington had not acceded to Protocol III and was not obliged to fulfill its requirements.
In 2006, the Israeli army used phosphorus against Lebanese residents. It is difficult to provide exact data on casualties. The Jewish state does not deny the fact of the use of chemical weapons in the Arab Republic.
The IDF reused phosphorus in 2009 when it conducted Operation Cast Lead in the Gaza Strip. According to the Western press, more than a hundred Palestinians became victims of incendiary shells.
Hamas militants also did not remain indifferent to white phosphorus. The substance was occasionally used to power Qassam rockets used by Palestinian resistance fighters to fire into Israeli territory during the 2009-2012 conflict.
Finally, 2020. Iraqi troops, supported by American forces, begin the siege of Fallujah, which is now occupied by Islamic State terrorists. Phosphorus shells are being used again. There are no reports of civilian casualties in Iraq's second largest city. Perhaps we will learn about them only after the end of the war.
Eduard Lukoyanov
Links
| phosphorus in Wiktionary |
| Phosphorus at Wikimedia Commons |
- Explosion of phosphorus with potassium nitrate (video) (inaccessible link from 04/14/2018 [179 days])
- Phosphorus Moon - White Phosphorus Experience
- Phosphorus, educational film
- Phosphorus on Webelements
- Phosphorus in the Popular Library of Chemical Elements
H
| Periodic table of chemical elements by D. I. Mendeleev | |||||||||||||||||||||||||||||||||||||||||||
| He | |||||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | MD | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||||||||||||
| Uue | Ubn | Ubu | Ubb | Ubt | Ubq | UBP | Ubh | ||||||||||||||||||||||||||||||||||||
| Alkali metals | Alkaline earth metals | Lanthanides | Actinoids | Superactinoids | Transition metals | Other metals | Semimetals | Other non-metals | Halogens | Noble gases | Properties unknown | ||||||||||||||||||||||||||||||||
Shortage
Symptoms of phosphorus deficiency in the human body can be similar to signs of calcium deficiency or magnesium deficiency. If the amount of one of the substances is reduced, the level of others should be checked. Bringing health back to normal requires an integrated approach.
The daily dose of organogen in the body is 1200 mg
. Lack of an element is determined by a number of signs. A medical examination can provide the most complete information.
Attention:
Phosphorus deficiency in childhood interferes with the normal growth of nerve fibers and brain cells, which impedes mental development.
Causes
It has been established that the decrease in phosphorus reserves does not occur without reason. Most often, this deviation is observed in obese people
.
There may be several reasons for the shortage. These include the following:
- Fasting or strict diets;
- Diseases accompanied by metabolic disorders;
- Poisoning;
- Excessive consumption of sugary carbonated drinks;
- Period of pregnancy or breastfeeding;
- An excess of substances such as calcium, barium, aluminum or magnesium.
Phosphorus deficiency often occurs in bottle-fed infants.
. Mother's milk contains much more nutrients necessary for the development of the baby. The microelements included in the mixture are not always absorbed properly.
People prone to display deviations include patients with diabetes, alcohol drinkers, patients suffering from hormonal disorders
. To prevent the development of unpleasant consequences, it is necessary to regularly monitor the level of the microelement.
On a note:
Phosphorus is partially removed from the body during urination.
Symptoms
mean:
- Painful sensations in bone tissue and muscles;
- Pathological changes in the myocardium;
- Decreased immunity;
- Development of periodontal disease;
- Mental illnesses.
Peculiarities:
a person suffering from phosphorus deficiency gradually loses vitality. He develops apathy and may experience depression.
Notes
- Michael E. Wieser, Norman Holden, Tyler B. Coplen, John K. Böhlke, Michael Berglund, Willi A. Brand, Paul De Bièvre, Manfred Gröning, Robert D. Loss, Juris Meija, Takafumi Hirata, Thomas Prohaska, Ronny Schoenberg, Glenda O'Connor, Thomas Walczyk, Shige Yoneda, Xiang-Kun Zhu.
Atomic weights of the elements 2011 (IUPAC Technical Report) // Pure and Applied Chemistry. — 2013. — Vol. 85, no. 5. - P. 1047-1078. — DOI:10.1351/PAC-REP-13-03-02. - Phosphorus: electronegativities (English). WebElements. Retrieved July 15, 2010.
- Sulfur and Phosphorus Compounds (English). Retrieved January 27, 2010. Archived August 22, 2011.
- ↑ 1 2 3 Editorial Board: Zefirov N. S. (chief editor).
Chemical encyclopedia: in 5 volumes. - Moscow: Great Russian Encyclopedia, 1999. - T. 5. - P. 145. - JP Riley and Skirrow G. Chemical Oceanography V. 1, 1965
- ↑ 1 2 3 Khodakov Yu. V., Epshtein D. A., Gloriozov P. A.
§ 30. Phosphorus // Inorganic chemistry. Textbook for 9th grade. — 7th ed. - M.: Education, 1976. - P. 62-65. — 2,350,000 copies. - Burning white phosphorus underwater - video experience in the Unified Collection of Digital Educational Resources
- Kemal T. Saracoglu, Ahmet H. Acar, Tamer Kuzucuoglu, Sezer Yakupoglu.
White phosphorus burn (English) // The Lancet. - 2010. - Vol. 376, no. 9734. - P. 68. - DOI:10.1016/S0140-6736(10)60812-4. - Chou TD, Lee TW, Chen SL, Tung YM, Dai NT, Chen SG, Lee CH, Chen TM, Wang HJ.
The management of white phosphorus burns (English) // Burns. - 2001. - Vol. 27, iss. 5. - P. 492-497. — DOI:10.1016/S0305-4179(01)00003-1. - PMID 11451604. - Radiation chemistry // Encyclopedic Dictionary of a Young Chemist. 2nd ed. / Comp. V. A. Kritsman, V. V. Stanzo. - M.: Pedagogy, 1990. - P. 200. - ISBN 5-7155-0292-6.
- W. Schröter, K.-H.
Lautenschläger, H. Bibrak et al. Chemistry = Chemie. - M.: Chemistry, 1989. - P. 351. - ISBN 5-7245-0360-3. - Chemical Encyclopedia / Editorial Board: Zefirov N.S. and others. - M.: Great Russian Encyclopedia, 1998. - T. 5. - 783 p. — ISBN 5-85270-310-9.
- Lidin R.A., Molochko V.A., Andreeva L.L.
Chemical properties of inorganic substances: Textbook. manual for universities. — 3rd ed., rev. - M.: Chemistry, 2000. - 480 p. — ISBN 5-7245-1163-0. - Data are given according to Audi G., Bersillon O., Blachot J., Wapstra AH
The NUBASE evaluation of nuclear and decay properties // Nuclear Physics A. - 2003. - T. 729. - P. 3-128. — DOI:10.1016/j.nuclphysa.2003.11.001. — Bibcode: 2003NuPhA.729….3A. - USDA
- Bulanov Yu. B.
Chemical composition of products. The nutritional value. - UNIAN - health - What happens to phosphorus?
Phosphorus - benefits, harm, daily intake and sources
The element is actively involved in processes such as the division and growth of cells in the body, as well as in the very important process of using and storing all the genetic information inherent in a person. It has a huge impact on the activity of many vitamins that are important for proper acid-base balance, and their benefits are undeniable.
The role of phosphorus in the human body is difficult to underestimate, and therefore it is very important to understand the danger of its deficiency and from which products the microelement is best absorbed by humans.