| This article violates the rule against instructions in encyclopedia articles. Its text almost completely repeats the instructions for use of the drug provided by its manufacturer. In addition, the data provided may not be sufficiently objective and reliable (especially regarding the effectiveness and safety of the drug). To maintain neutrality of presentation, it is necessary to rework this article based on independent authoritative sources of information. |
| Budesonide | |
| Budesonide | |
| Budesonide | |
| Chemical compound | |
| IUPAC | (11β, 16α)-16,17-(butylidene-bis(oxy)-11,21-dihydroxypregna-1,4-diene-3,20-dione |
| Gross formula | C₂₅H₃₄O₆ |
| Molar mass | 430.534 g/mol |
| CAS | 51333-22-3 |
| PubChem | 63006 |
| DrugBank | APRD00442 |
| Classification | |
| ATX | A07EA06 D07AC09, R01AD05, R03BA02 |
| Pharmacokinetics | |
| Bioavailable | 100% (but large first pass effect) |
| Plasma protein binding | 85-90 % |
| Metabolism | Hepatic CYP3A4 |
| Half-life | 2.0-3.6 hours |
| Excretion | Renal, Faecal |
| Dosage forms | |
| metered aerosol for inhalation, capsules with powder for inhalation, metered powder for inhalation, solution for inhalation, metered suspension for inhalation, nasal drops, metered nasal spray, capsules, ointment for external use | |
| Other names | |
| Apulein, Benacort, Benarin, Budecort, Budenofalk, Buderin, Budesonide, Budesonide Easyhaler, Budesonide powder for inhalation, Pulmicort, Pulmicort Turbuhaler, Tafen nasal, Tafen Novolizer, Cicortide Cyclocaps | |
| Budesonide at Wikimedia Commons | |
Budesonide
- a drug, glucocorticoid, has anti-inflammatory, antiallergic and immunosuppressive effects.
Budesonide is included in the list of vital and essential drugs.
pharmachologic effect
GCS for inhalation, intranasal and local use. It has an anti-inflammatory, anti-allergic and anti-exudative effect, which, when used in inhalation, leads to a reduction in bronchial obstruction. The mechanism of action is to inhibit the release of inflammatory and allergic mediators, as well as to reduce the reactivity of the respiratory tract to their action. During the treatment, respiratory function improves, the severity and frequency of shortness of breath, attacks of suffocation, and cough are significantly reduced. The maximum clinical effect develops after 1-2 weeks of therapy.
Indications
- For spray:
Seasonal and year-round allergic rhinitis. For spray (optional) - vasomotor rhinitis; prevention of the growth of nasal polyps after polypectomy; non-infectious inflammatory processes in the nasal cavity. - For capsules:
Crohn's disease (mild and moderate forms involving the ileum and/or ascending colon). - For ointment:
Allergic dermatitis, psoriasis, eczema; lichen planus, atopic dermatitis. - For inhalations:
Bronchial asthma (as a basic therapy; with insufficient effectiveness of beta2-adrenergic stimulants, cromoglycic acid and ketotifen; to reduce the dose of oral corticosteroids), chronic obstructive pulmonary disease. - For ampoules:
With saline solution for rinsing the nasal cavity.
special instructions
Use with caution in patients with pulmonary tuberculosis, as well as chickenpox.
Budesonide is not intended for the relief of acute attacks of asthma in bronchial asthma. Use is not recommended for intermittent disease.
The replacement of systemic corticosteroids with inhalations is carried out gradually.
The effectiveness and safety of budesonide in children under 7 years of age have not been studied.
Impact on the ability to drive vehicles and operate machinery
Budesonide does not affect the ability to engage in potentially hazardous activities that require increased attention and high speed of psychomotor reactions.
Instructions:
Clinical and pharmacological groups
12.006 (Drug with anti-inflammatory and bronchodilator action) 11.066 (GCS for oral use. Drug for the treatment of Crohn's disease) 04.008 (GCS for intranasal use) 04.005 (GCS for inhalation)
pharmachologic effect
GCS for inhalation, intranasal and local use. It has an anti-inflammatory, anti-allergic and anti-exudative effect, which, when used in inhalation, leads to a reduction in bronchial obstruction. The mechanism of action is to inhibit the release of inflammatory and allergic mediators, as well as to reduce the reactivity of the respiratory tract to their action. During the treatment, respiratory function improves, the severity and frequency of shortness of breath, attacks of suffocation, and cough are significantly reduced. The maximum clinical effect develops after 1-2 weeks of therapy.
Pharmacokinetics
When administered by inhalation, 30-35% of the dose penetrates the bronchioles. Bioavailability when entering the lungs is about 73%. 25-30% enter the gastrointestinal tract. Bioavailability by oral route is 10.7%.
Cmax of budesonide in plasma is achieved after 1.5-2 hours.
Budesonide is quickly eliminated from the body. T1/2 for inhalation administration is 2-3 hours. Plasma clearance is 55-85 l/hour.
Dosage
For inhalation use, the dose is set depending on the indications, age, and method of inhalation.
Drug interactions
Cimetidine and omeprazole do not have a clinically significant effect on the pharmacokinetic parameters of budesonide when taken orally. However, under the influence of cimetidine, the metabolism of budesonide in the liver may slow down.
Use during pregnancy and lactation
Use during pregnancy is possible when the expected benefit to the mother outweighs the potential risk to the fetus.
If use is necessary during lactation, breastfeeding should be discontinued due to the lack of data on the excretion of budesonide in breast milk.
Side effects
From the respiratory system: when used in inhalation, irritation of the mucous membranes of the pharynx, oral cavity, nose, and candidiasis is possible; with increased sensitivity - bronchospasm.
Indications
Bronchial asthma requiring maintenance therapy with glucocorticoids.
Contraindications
Hypersensitivity to budesonide.
special instructions
Use with caution in patients with pulmonary tuberculosis, as well as chickenpox.
Budesonide is not intended for the relief of acute attacks of asthma in bronchial asthma. Use is not recommended for intermittent disease.
The replacement of systemic corticosteroids with inhalations is carried out gradually.
The effectiveness and safety of budesonide in children under 7 years of age have not been studied.
Impact on the ability to drive vehicles and operate machinery
Budesonide does not affect the ability to engage in potentially hazardous activities that require increased attention and high speed of psychomotor reactions.
Preparations containing BUDESONIDE
• BUDENIT STERI-NEB susp. for inhalation 500 mcg/1 ml: amp. 2 ml 20, 30 or 60 pcs. • BENACORT® (BENACORT) solution for inhalation. 500 mcg/1 ml: vial. 2.2 ml 10 pcs. • SYMBICORT® TURBUHALER® powder for inhalation dosed 160 mcg+4.5 mcg/1 dose: turbuhaler 60 or 120 doses • BENACORT® powder for inhalation. 200 mcg/1 dose: Cyclohaler inhalers 100 doses or 200 doses • BIASTEN® powder for inhalation dosage. 100 mcg+400 mcg/1 dose: Cyclohaler inhalers 100 doses or 200 doses • PULMICORT® suspension. d/inhal. dosed 1 mg/2 ml: single-dose containers 20 pcs. • FORADIL® COMBI set of capsules. with powder for inhalation: 40, 60, 70, 90, 120, 150 or 180 pcs. in a pack, including: caps. 12 mcg formoterol: 30 or 60 pcs.; caps. 400 mcg budesonide: 10, 30, 60 or 120 pcs. included with a device for inhalation (aerolyzer) • PULMICORT® TURBUHALER® powder for inhalation. dosage 200 mcg/1 dose: turbuhaler 100 or 200 doses • BENACORT® (BENACORT) solution for inhalation. 250 mcg/1 ml: vial. 2.2 ml 10 pcs. • BUDESONID EASYHALER powder for inhalation. dosed 200 mcg/1 dose: inhalers 200 doses • TAFEN® NOVOLIZER powder for inhalation. dosage 200 mcg/1 dose: cartridge 200 doses (included with or without an inhaler) • BIASTEN® powder for inhalation dosage. 100 mcg+100 mcg/1 dose: Cyclohaler inhalers 100 doses or 200 doses • BUDIAIR aerosol d/inhaler. dosed 200 mcg/1 dose: vial. 200 doses • FORADIL COMBI (FORADIL® COMBI) set of caps. with powder for inhalation: 40, 60, 70, 90, 120, 150 or 180 pcs. in a pack, including: caps. 12 mcg formoterol: 30 or 60 pcs.; caps. 200 mcg budesonide: 10, 30, 60 or 120 pcs. included with an inhalation device (aerolyzer) • BUDENIT STERI-NEB suspension. for inhalation 250 mcg/1 ml: amp. 2 ml 20, 30 or 60 pcs. • CYCORTIDE CYCLOCAPS powder for inhalation. in capsules 200 mcg: 30, 60, 100 or 120 pcs. • SYMBICORT® TURBUHALER® powder for inhalation, dosed 320 mcg+9 mcg/1 dose: turbuhaler 60 doses • BIASTEN® powder for inhalation, dosage. 100 mcg+200 mcg/1 dose: Cyclohaler inhalers 100 doses or 200 doses • PULMICORT® suspension. d/inhal. dosed 500 mcg/2 ml: single-dose containers 20 pcs. • PULMICORT® TURBUHALER® powder for inhalation. dosage 100 mcg/1 dose: turbuhaler 200 doses • BENARIN nasal drops 0.05%: vial. 5 ml or 10 ml, dropper bottles 5 ml or 10 ml • BUDENOFALK caps. 3 mg: 20, 50 or 100 pcs. • SYMBICORT® TURBUHALER powder for inhalation dosed 80 mcg+4.5 mcg/1 dose: turbuhalers 60 or 120 doses • TAFEN® NASAL nasal spray dosed 50 mcg/1 dose: vial. 200 doses per dosage. device
Additional recommendations
You should not drink alcohol while using the substance, as this can lead to a decrease in the effectiveness of the therapeutic effect and the development of side effects in the stomach, and an exacerbation of the existing disease.
Budesonide can be used as therapy even if other similar medications are prohibited due to adverse reactions.
To prevent the development of candidiasis, after taking the product you need to constantly rinse your mouth after use.
It is necessary to protect your eyes from getting the product into them.
When switching from one form to another, you need to consult a doctor and do everything gradually under his supervision.
Due to long-term use, pseudorheumatism syndrome may develop, which will most likely lead to an increase in dosage, but on a temporary basis, and only a doctor can say this.
There may be a slight pain in the head, increased fatigue, nausea, and vomiting.
When treatment is stopped, it is necessary to be observed by a doctor in order to timely establish the onset of the development of adrenal insufficiency and evaluate the functions of external respiration.
You should not self-medicate and prescribe the drug without a doctor. Only by following this rule can you avoid harming yourself.
Budesonide release form
Since Budesonide is not only an active ingredient and a non-patented international name for drugs, there are several dosage forms of the drug:
1
Budesonide-Nativ - in addition to the active ingredient, contains macrogol, water, succinic acid, pripylene glycol, nipagin. Available in the form of a colorless or dim yellow solution for inhalation. Not for use in children under 16 years of age.
2
Budesonide Easyhaler is a special powder for inhalation, which contains 200 mcg of local corticosteroid and lactose monohydrate. Not suitable for children.
3
Pulmicort is a dosed suspension for the treatment of chronic obstructive diseases. Available in bottles for preparing a solution or directly in aerosol inhalers. One dose may contain 500 or 250 mcg of the active ingredient.
4
Budesonide Steri-Neb is another suspension that comes in small polyethylene ampoules and is white in color. The composition includes water, citric acid, sodium chloride, polysorbate, sodium citrate. 1 ml of suspension can contain 0.5 or 0.25 hormone.
Consequently, the drug is produced by pharmaceutical companies in the form of a suspension, powder and ready-made solution.
Directions for use and doses
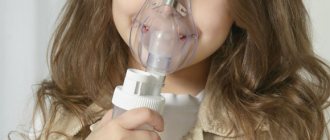
Inhalation:
the dose is selected individually, taking into account the severity of the disease and the maintenance dose of tableted glucocorticoids; adults - 200-800 mcg/day (up to 1600 mcg/day), in several doses; for children - depending on age.
Inside:
without chewing (if swallowing is impaired, you can open the capsule and swallow all the granules), with a sufficient amount of liquid (a full glass of water), 30 minutes before meals, 3 mg 3 times a day (morning, noon and evening). The course of treatment is usually 8 weeks; As a rule, the effect appears after 2-4 weeks. Cancellation is carried out gradually.
Intranasally:
the dose and duration of treatment are selected individually, taking into account the severity of the disease; for adults and children over 18 years of age, a single dose is 2-3 drops in each nasal passage; As a rule, 2 single doses per day are sufficient; Course duration is 10-14 days.
Interaction
Cytochrome P450 inhibitors (including ketoconazole, erythromycin, cyclosporine) may slow metabolism and enhance the glucocorticoid effect. Budesonide may enhance the effect of cardiac glycosides (due to potassium deficiency); saluretics may increase hypokalemia. The simultaneous administration of cimetidine and budesonide may lead to a slight increase in plasma budesonide levels (no clinical significance). Omeprazole (when administered concomitantly) does not affect the pharmacokinetics of budesonide.
Theoretically, interaction with resins that can bind steroids (for example cholestyramine), as well as antacids, cannot be excluded. When these drugs are taken concomitantly, interactions may reduce the therapeutic effect of budesonide. In this regard, the above drugs should be taken at least 2 hours apart [1].
Reception, prohibition of substances
When buying Budesonide, you need to check that there is an instruction manual that describes the main diseases for which this remedy is recommended:
- Bronchial asthma – removes inflammation and inhibits active inflammatory mediators.
- Crohn's disease - thanks to the pills, remission is induced, but only if the disease is mild or moderate in severity.
- Rhinitis - an inhalation solution relieves symptoms of hay fever and allergies.
- Recurrence of the development of polyposis in the nasal mucosa - prevention at an early stage prevents new formation of the disease.
- Preventive process in the development of obstructive pulmonary disease.
It is important to remember that the dose portion is very important so as not to harm the adrenal glands and not activate glucocorticoid withdrawal syndrome.
A similar effect is observed with Symbicort Turbuhaler.
Budesonide for inhalation, as well as its other forms, has contraindications, and these are the following:
- Intolerance to the components of the substance, greater sensitivity to it, which can lead to angioedema and disruption of hormonal properties in the body.
- Pulmonary tuberculosis.
- Fungal respiratory disease.
- Acute infections in the stomach.
- Liver failure.
- Budesonide is contraindicated in children.
If at least one of these contraindications exists, then the use of the medicine is prohibited.
Children should not take this medicine, as it causes disruption of glucocorticoid hormone production.
When using the product, those with arterial hypertension, diabetes mellitus, pheochromocytoma, callous ulcers of the stomach and duodenum should be careful.
It should be taken carefully during pregnancy and breastfeeding and only on the recommendation of a doctor, and if the benefits are greater than the harm.
Budesonide for inhalation for children
Most often, children are prescribed Pulmicort, which is allowed from 6 months of age. How to dilute budesonide for inhalation with saline solution: add 1 to 4 ml of the medication along with 1-2 ml of saline solution into the nebulizer.
Children from 6 months are prescribed 0.25-0.5 mg (1-2 ml per day). The maximum permissible dosage for a child is 2 mg per day.
The instructions say that if the dose is not more than 1 mg, it can be administered at a time.