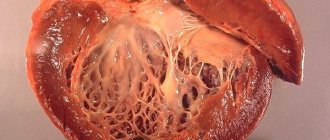
Ischemic cardiomyopathy is a myocardial disease characterized by an increase in the size of the heart cavities and clinical symptoms of CHF, caused by atherosclerotic lesions of the coronary arteries. In foreign medical literature, ischemic dilated cardiomyopathy is understood as a myocardial disease, characterized by an enlargement of all chambers of the heart to the degree of cardiomegaly, with uneven thickening of its walls and phenomena of diffuse or focal fibrosis, developing against the background of atherosclerotic lesions of the coronary arteries.
In ICD-10, ischemic cardiomyopathy is presented in class IX “Diseases of the circulatory system” in heading I 25.5 as a form of chronic ischemic heart disease. In the classification of cardiomyopathy (WHO/MOFC, 1995), ischemic cardiomyopathy is classified as a group of specific cardiomyopathies. Ischemic dilated cardiomyopathy is a myocardial lesion caused by diffuse, significant atherosclerosis of the coronary arteries, manifested by cardiomegaly and symptoms of congestive heart failure. Patients with ischemic dilated cardiomyopathy make up about 5-8% of the total number of patients suffering from clinically significant forms of coronary artery disease. Among all cases of cardiomyopathies, ischemic ones account for about 11-13%. Ischemic cardiomyopathy occurs mainly at the age of 45-55 years; among all patients, men make up 90%.
Causes of ischemic cardiomyopathy
The first place among the provocateurs of ischemic cardiomyopathy is occupied by atherosclerosis, which slowly but surely affects the coronary arteries - the main vessels of the heart muscle. As a result, there is a significant slowdown in blood flow in the heart, as the lumen of the vessel narrows. The patient develops hypertrophy and intramyocardial pressure increases.
Often, ischemic cardiomyopathy develops as a result of a previous myocardial infarction. Especially if a repeated or recurrent attack occurs. Another cause of ischemic cardiomyopathy is angina.
More rare causes of pathology include metabolic disorders, which occur with endocrine diseases, sudden weight gain or loss, as well as during menopause.
Combining all the reasons for the development of ischemic cardiomyopathy, we can name the factors that provoke their appearance:
- bad habits;
- heredity;
- diabetes;
- high concentration of cholesterol in the blood;
- hypertension.
Causes of ICMP
Ischemic cardiomyopathy (ICMP) manifests itself in the form of damage to the coronary arteries, which is caused by their narrowing due to atherosclerosis. Such a metabolic failure in the body leads to the appearance of a solid substance in the arteries of atherosclerotic plaques, which impede blood flow and the proper functioning of the entire vascular system.
The heart muscle weakens and stops working actively, which leads to a deterioration in blood pumping. The patient begins to have attacks and symptoms of angina pectoris appear.
Most doctors agree that the etiology of dilated heart disease is influenced by the following factors:
- predisposition at the genetic level, inherited,
- high blood pressure,
- unhealthy lifestyle: overeating, non-compliance with the daily routine, lack of physical activity to keep the body in shape,
- pathology of the thyroid gland,
- high levels of lipids in blood plasma,
- pathological changes in the adrenal cortex.
How does ischemic cardiomyopathy develop?
When a certain portion of blood is released, the cardiac cavities stretch, which causes their dilatation. In addition, ventricular remodeling is possible, resulting in the formation of fibrosis. And diffuse fibrosis is a direct path to the development of heart failure due to cardiomyopathy.
It is worth noting that hyperfusion of the LV (left ventricle) also often causes the development of cardiomyopathy, since the contractile function in it is reduced. In this case, we are talking about myocardial hibernation.
Another significant cause of the development of ischemic cardiomyopathy is myocardial apoptosis, which consists of programmed cell death. This disorder is characteristic of ischemic disease. The development of ischemic cardiomyopathy is also influenced by an increased concentration of cytokines.
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy is considered a disease of unknown cause. However, more than 50 genes have now been identified whose mutations cause disruption of protein structure in myocardial cells. The appearance of such genetic changes leads to the development of this disease. These mutations are inherited in an autosomal dominant manner. This means that a sick parent is very likely to have a sick child.
Among the entire population, hypertrophic ICM is observed in 2–5 people out of 10,000. A quarter of patients develop obstructive hypertrophic ICM, the rest develop its non-obstructive form. Manifestations of the disease depend primarily on diastolic dysfunction of the heart, that is, on the impairment of its ability to relax. The main symptoms include shortness of breath, dizziness and fainting, heart pain as a result of myocardial ischemia and heart rhythm disturbances (extrasystole, paroxysmal supraventricular tachycardia, various blockades).
When planning pregnancy, women with fainting conditions or severe arrhythmias need to consider surgery for hypertrophic cardiomyopathy.
Read also: Exercise for cardiomyopathy
For most women, pregnancy does not adversely affect circulation. The main danger is the development of severe heart rhythm disturbances.
If the disease is mild, the patient should be examined by a cardiologist once a month. She is hospitalized at a time that is usual for all pregnant women with cardiovascular diseases. Childbirth takes place through the natural birth canal.
If the outflow tract of the left ventricle is moderately narrowed, individual monitoring is carried out. Hospitalization periods are usual, more often if necessary. Childbirth can be natural, but with the exception of pushing.
With severe obstruction (narrowing) of the outflow tract and a pressure gradient of 50 mm Hg. Art. and above pregnancy is contraindicated. If a woman insists on continuing the pregnancy, hospitalization is carried out for almost the entire period of bearing the child. Delivery occurs via caesarean section. In this case, epidural anesthesia must be performed with great caution due to the risk of vasodilation and a sharp drop in blood pressure.
For drug treatment of arrhythmias, beta-blockers or verapamil are most often prescribed. Quite often it is necessary to resort to surgical treatment: removal of part of the interventricular septum that prevents normal blood flow. As a result, in many cases, complete atrioventricular block develops, requiring the installation of a pacemaker.
Characteristic symptoms of ischemic cardiomyopathy
Signs characteristic of ischemic cardiomyopathy include:
- congestive heart failure (noted in 100% of cases);
- angina pectoris (determined in most patients);
- thromboembolic complications;
- shortness of breath, which bothers you even at rest;
- attacks of suffocation that occur at night;
- weight gain;
- swelling of the legs, urinary retention;
- severe weakness and fatigue.
Cardiomyopathy develops very slowly, as a result of which patients do not observe obvious deviations in their health and, accordingly, do not seek medical help.
Less commonly, the following symptoms may occur:
- angina attacks that occur steadily;
- mild pain in the right hypochondrium;
- tachycardia;
- arrhythmia (various forms);
- lack of appetite.
The insidiousness of the symptoms of ischemic cardiomyopathy lies in their attack-like nature and infrequent manifestation. Therefore, in most cases, patients consult a doctor when the disease has progressed.
Restrictive cardiomyopathy
The name of the disease comes from the Latin word meaning restriction. This disease is manifested by a significant impairment of diastolic myocardial function. This means that the heart does not relax well, the walls do not stretch, the volume of the ventricles in diastole does not increase, and the pumping function is sharply limited. Heart failure develops.
Myocardial cells die under such conditions and are replaced by connective tissue. The heart valves are also affected. Severe heart rhythm disturbances develop. Restrictive CMP is a rare condition. Most often it occurs as a complication of other diseases - amyloidosis, hemochromatosis, endomyocardial fibrosis, eosinophilic Loeffler's endomyocarditis. The development of this disease is likely under the influence of ionizing radiation, as well as intoxication, in particular, anthracyclines.
Restrictive CMP is a contraindication for pregnancy.
Diagnosis of ischemic cardiomyopathy
First, the cardiologist performs a physical examination. At the same time, it determines the presence of heart murmurs. Further diagnosis of the patient is carried out using laboratory and instrumental methods.
Laboratory diagnostic methods mean a blood test that will help identify the level of triglycerides, lipoproteins, and cholesterol in the blood.
As for instrumental examination methods, these include:
- Electrocardiography is a method that allows you to diagnose coronary heart disease and its numerous other lesions.
- Daily monitoring is a very important diagnostic method, since it allows you to identify pathologies at the very beginning of development, before any symptoms appear.
- Echocardiography allows visualization of the heart. This method determines hypertrophy, enlargement of the heart cavities and many other pathologies.
- Coronary angiography, which allows you to determine the condition of the coronary vessels and identify atherosclerotic plaques in them.
- PET (positron emission tomography) is performed to evaluate metabolic changes in the myocardium.
- MRI (magnetic resonance imaging) is used when another diagnostic method is not sufficiently informative.
Instrumental diagnostics reveals any morphological changes in the heart.
Peripartum (postpartum) cardiomyopathy
What is peripartum cardiomyopathy, what are the clinical manifestations of the disease? What methods are used for treatment?
Peripartum cardiomyopathy (PPCM) is a rare disease but is associated with significant maternal mortality. PPCM is a life-threatening disease of unknown etiology that develops in previously healthy women during the peripartum period and is characterized by the development of left ventricular (LV) systolic dysfunction and symptomatic heart failure (HF) during the last month of pregnancy or 5 months after delivery.
According to the definition of the European Society of Cardiology, PPCM is a form of dilated cardiomyopathy (DCM), which is characterized by the development of signs of heart failure during the last
months of pregnancy or the first 5 months after birth. PPCM is relatively rare. The incidence of PPCM ranges from 1 case in 1300 to 1 case in 15 thousand women who gave birth to live children [68] (on average, 1 case in 2289 women giving birth).
Risk factors for developing PPCM include:
- age. PPCM can develop in women of any age, but the highest incidence is observed in pregnant women over 30 years of age;
- number of pregnancies and births. Multiparous women are considered to be at risk for PPCM;
- multiple pregnancy;
- gestational hypertension. According to the literature, the incidence of gestational arterial hypertension (AH) in patients with PPCM ranges from 8–10% [14] to 43%;
- preeclampsia, which is diagnosed in 22% of patients with PPCM;
- ethnic representation;
- genetic predisposition;
- tocolytic therapy;
- cocaine use.
The mechanisms of cardiomyocyte damage in PPCM are not completely clear. Their damage can develop as a result of viral infection, genetic disorders and toxemia involving autoimmune mechanisms. There is evidence of the etiological role of viral myocarditis in the development of DCM both in animal experiments and in humans.
The role in the pathogenesis of PPCM of prolactin, relaxin, the formation of immune complexes, cardiac NO synthetase, immature dendritic cells, cardiac dystrophin, toll-like receptors, selenium deficiency, which increases the sensitivity of the myocardium to damaging influences and activation of oxidative stress is considered. .
Clinical manifestations of PPCM
Clinical manifestations of PPCM are caused by the development of LV systolic dysfunction and are similar to those in idiopathic DCM.
Symptoms such as shortness of breath, dizziness, swelling of the lower extremities can also be observed during a normal pregnancy. Shortness of breath during pregnancy is associated with hyperventilation caused by progesterone, as well as the pressure of the growing uterus on the diaphragm. Approximately two thirds of healthy pregnant women have peripheral edema. However, if edema and other symptoms of HF suddenly appear, PPCM must be excluded.
Read also: Cardiomyopathy in children treatment
Clinical manifestations of PPCM, in addition to symptoms of congestive heart failure, may include cardiac arrhythmias and thromboembolic complications. During pregnancy, the risk of thromboembolic complications increases due to high concentrations of blood coagulation factors II, VII, VIII, X and fibrinogen, and may also be associated with dilatation of the cardiac cavities, systolic dysfunction and the presence of atrial fibrillation. The increased risk of their occurrence may persist for up to 6 weeks after birth.
Cases of asymptomatic progression of this disease have also been described. The diagnosis of PPCM is a diagnosis of exclusion.
What is the difference between ischemic cardiomyopathy and dilated cardiomyopathy?
Ischemic and dilated cardiomyopathies have similar symptoms, but different approaches to treatment. Therefore, the first step is to carry out a differential diagnosis between these two pathologies.
Their general clinical picture is as follows:
- the presence of areas where cardiac contractility is impaired;
- cicatricial changes in the heart muscle;
- increase in heart size;
- developing failure.
If we are talking about the ischemic form of pathology, then the following signs are more typical for it:
- diffuse cardiosclerosis and stenosis of the coronary arteries, which are detected during coronary angiography and ECG;
- typical manifestations of angina pectoris;
- previous myocardial infarction.
In the dilated form of cardiomyopathy, embolism and thrombosis occur in 60% of patients, while in ischemic pathology these complications occur in 40%.
Diagnosis of the disease Ischemic dilated cardiomyopathy
LABORATORY AND INSTRUMENTAL DIAGNOSTICS
Blood chemistry
Characterized by an increase in the blood levels of total cholesterol, low-density lipoprotein cholesterol, and triglycerides, which is characteristic of atherosclerosis.
Electrocardiography
Cicatricial changes may be detected after previous myocardial infarctions or signs of ischemia in the form of horizontal displacement downwards from the ST interval isoline in various parts of the myocardium. Many patients exhibit nonspecific diffuse changes in the myocardium in the form of a decrease or smoothing of the T wave. Sometimes the T wave is negative, asymmetrical or symmetrical. Signs of myocardial hypertrophy of the left ventricle or other parts of the heart are also characteristic. Various arrhythmias (usually extrasystole, atrial fibrillation) or conduction disturbances are recorded. Daily Holter ECG monitoring often reveals silent, painless myocardial ischemia.
Echocardiography
EchoCG reveals dilatation of the cardiac cavities, slight myocardial hypertrophy, an increase in end-diastolic volume, diffuse hypokinesia of the walls of the left ventricle, and a decrease in ejection fraction. The ejection fraction of the right ventricle in patients with ischemic cardiopathy, compared with the ejection fraction of the left ventricle, is reduced to a lesser extent than in idiopathic dilated cardiomyopathy.
In the presence of chronic myocardial ischemia, the rigidity and rigidity of the walls of the left ventricle significantly increases, and their elasticity decreases. This is due to a deficiency of high-energy compounds due to insufficient oxygen supply to the myocardium. Which leads to a slowdown in the process of early diastolic relaxation of the left ventricular myocardium. These circumstances lead to the development of the diastolic form of heart failure. Left ventricular diastolic dysfunction in coronary artery disease can occur without impairment of systolic function.
According to Doppler echocardiography, there are two main types of left ventricular diastolic dysfunction - early and restrictive. The early type is characterized by a violation of the early phase of diastolic filling of the left ventricle. During this phase, the speed and volume of blood flow through the mitral orifice decrease (peak E) and the volume and speed of blood flow increase during atrial systole (peak A). The time of isometric relaxation of the left ventricular myocardium increases and the time of deceleration of the E flow increases, the E/A ratio < 1. With the restrictive type of diastolic dysfunction of the left ventricle, the diastolic pressure in it significantly increases, the pressure in the left atrium increases, the E peak increases, the A peak decreases, and shortens left ventricular isometric relaxation time and flow deceleration time E, E/A ratio > 2.
With ischemic cardiomyopathy, the development of diastolic dysfunction is possible; the restrictive type is observed much less frequently. With the development of isolated diastolic HF, left ventricular systolic function is preserved and the ejection fraction is normal. With ischemic cardiomyopathy, isolated diastolic failure is rare; more often, with severe congestive heart failure, we are talking about combined systolic and diastolic dysfunction of the left ventricle.
X-ray examination
Determines a significant increase in the size of all chambers of the heart.
Radioisotope scintigraphy
Detects small foci of impaired accumulation of thallium-201 in the myocardium, which reflects myocardial ischemia and fibrosis.
Coronary angiography
Detects significantly pronounced atherosclerotic lesions of the coronary arteries. In this case, one of the arteries can be narrowed by more than 50%.
DIAGNOSTIC CRITERIA
The diagnosis of the disease is made on the basis of the above clinical picture and data from instrumental studies. First of all, the presence of angina pectoris, anamnestic data on myocardial infarction, cardiomegaly, and congestive heart failure are taken into account. Diagnosis of ischemic dilated cardiomyopathy is based on the diagnostic criteria outlined in Table 9.
Table 9. Diagnostic criteria for ischemic dilated cardiomyopathy
| Diagnostic criteria | Notes on criteria |
| 1. The presence of angina pectoris currently or in the past, or previous myocardial infarction, which precede the development of chronic heart failure | The criterion is confirmed by ECG, EchoCG. Angina pectoris or a previous myocardial infarction does not always precede the development of CHF; painless ischemia may be present long before the clinical manifestations of ischemic cardiomyopathy appear. Sometimes previous attacks of angina disappear or weaken |
| 2. Cardiomegaly | Determined by cardiac percussion, but must be verified using echocardiography |
| 3. The presence of clinical and echocardiographic signs of heart failure (decreased ejection fraction, increased end-diastolic volume and pressure, diffuse myocardial hypokinesia) | In severe congestive heart failure, there is usually combined systolic heart failure (LVEF) and left ventricular diastolic dysfunction (Doppler sonographic evidence of impaired diastolic filling of the left ventricle) |
| 4. Detection of areas in the myocardium that are in a state of hibernation | Methods used: stress echocardiography with dobutamine, positron emission tomography with fluorine-fluorodeoxyglucose; myocardial scintigraphy with 100T1 and 221Tc and comparison of areas of impaired isotope accumulation with areas of asynergy identified by echocardiography |
| 5. Detection during coronary angiography of a pronounced atherosclerotic process with narrowing of the lumen of one of the main | |
| 6. Absence of ventricular aneurysm and organic pathology of the valve apparatus, other causes of cardiomegaly |
DIFFERENTIAL DIAGNOSTICS
It is necessary to differentiate ischemic cardiomyopathy from various types of dilated cardiomyopathy. Diagnostic criteria for individual forms of dilated cardiomyopathy are presented in the relevant chapters. Table 10 shows the differential diagnostic signs of ischemic and idiopathic cardiomyopathies.
Table 10. Differential diagnostic signs of idiopathic and ischemic dilated cardiomyopathies
| Signs | Idiopathic dilatational cardiomyopathy | Ischemic dilatational cardiomyopathy |
| Age of patients | Typically young (20-40 years old) | Usually 50-55 years and older |
| History of angina or myocardial infarction | None | Characteristic |
| Relationship between the onset of clinical manifestations of heart failure and previous infection | Observed in 1/3 of patients | Uncharacteristic |
| Family nature of the disease | Can be traced in 20-30% of cases | Very rarely seen |
| Current presence of angina attacks | Not typical | Characteristic |
| Silent myocardial ischemia | Rarely | Often |
| Time from the onset of the HF clinic to death | 4-7 years (except for the course with progression of the disease and death within 1-2 years) | Typically less than 5 years |
| Thromboembolic syndrome | In 60% of cases | In 40% of cases |
| Atrial fibrillation | In 35-40% of cases | In 15-20% of cases |
| Atrioventricular block | In 30-40% of cases | In 10-15% of cases |
| Bundle branch blocks | In 30-40% of cases | In 10-15% of cases |
| Left ventricular dilatation | Sharply expressed | Sharply expressed |
| Right ventricular dilatation | Characteristic, significantly expressed | Less pronounced |
| Presence of zones of local myocardial hypokinesia during echocardiography | Less typical, more often diffuse myocardial hypokinesia | myocardial infarction |
| Echocardiographic and radiological signs of aortic atherosclerosis | Uncharacteristic, sometimes found, mildly expressed | Characteristic, always found, significantly expressed |
| Decreased left ventricular ejection fraction | Characteristic | Characteristic |
| Increased right ventricular ejection fraction | More pronounced than with ischemic cardiomyopathy | Less pronounced than with idiopathic dilated cardiomyopathy |
| Presence of atherogenic hyperlipoproteinemia | Not typical | Very characteristic |
| Presence of signs of atherosclerosis during external examination of the patient | Not typical | Characteristic |
Treatment
Treatment of ischemic cardiomyopathy begins with eliminating the main cause of its development – coronary heart disease.
Drug treatment consists of taking medications that improve cardiac function, prevent complications, and reduce symptoms. Depending on the situation and condition of the patient, the doctor prescribes the following groups of medications:
- calcium channel blockers, which lower blood pressure and dilate coronary arteries;
- Aldosterone inhibitors are designed to reduce fluid in the body. The drugs eliminate symptoms such as swelling and shortness of breath, and also reduce blood pressure;
- beta blockers are intended to stabilize blood pressure and reduce heart rate;
- diuretics are used to remove excess fluid, which, in turn, reduces the amount of circulating blood. Thus, the load on the heart decreases and blood pressure returns to normal;
- drugs that reduce blood clotting;
- medications that control heart rhythm.
Diagnostics
A diagnosis of PPCM with severely reduced left ventricular ejection fraction (≤35%) appears to increase the risk of ventricular tachyarrhythmias early after diagnosis. The use of wearable cardioverters/defibrillators may be considered in women with this condition within the first 6 months of starting treatment for heart failure.
Differential diagnosis includes the following:
- Aortic stenosis
- Cardiomyopathy, Alcohol
- Cardiomyopathy, dilated
- Cardiomyopathy, hypertrophic
- Cardiomyopathy, Restrictive
- Cardiovascular disease and pregnancy
- Atherosclerosis of the coronary artery
- high blood pressure
- Hypertension and pregnancy
- Hypertension, Malignant
- Mitral stenosis
- Non-cardogenic pulmonary edema during pregnancy. Pregnancy is a state of low oncotic pressure, reflected by a decrease in serum albumin (expected values, ~3.2 mg/dL); therefore, when other stressors are present, pulmonary edema may occur at normal cardiac filling pressures; most common triggers include pyelonephritis and other infections, corticosteroids and tocolytics such as beta-agonists and magnesium sulfate
- Preeclampsia (toxemia during pregnancy)
- Lung disease and pregnancy
- Pulmonary edema, cardiogenic
- Pulmonary edema, neurogenic
Other issues to consider include the following:
- Arrhythmogenic right ventricular dysplasia
- Cardiomyopathy, diabetic heart disease
- Infectious, toxic or metabolic disorders
Surgical methods of treatment
Ischemic cardiomyopathy in severe cases requires surgical intervention. These methods include:
- implantation of a pacemaker, which is carried out to normalize the heart rhythm;
- angioplasty, designed to dilate affected vessels;
- stents are special tubes that keep arteries open;
- atherectomy - this method of surgical intervention allows you to remove plaque from the venous walls;
- Radiation therapy is used to keep the arteries clear after angioplasty.
In some cases, coronary artery bypass grafting is used. This method is carried out to improve blood flow to the heart muscle. To do this, a previously removed vein is placed near the affected artery. This is necessary so that the blood moves through the new vessel, bypassing the affected one.
If we are talking about too large a lesion, then the only method to save the patient’s life is a heart transplant.
Severe ischemic cardiomyopathy: there is not much chance of survival without help
Insufficient blood flow to the heart muscle leads to coronary artery disease.
Its complication may be cardiomyopathy, in which the myocardium weakens to such an extent that it loses tone, and the chambers of the heart expand. Weak cardiac output provokes circulatory failure.
With extensive damage to the heart muscle, death can occur if it is impossible to perform a heart transplant.
Causes of ICMP development and risk factors
Cardiomyopathy occurs when the coronary arteries are damaged, most often of atherosclerotic origin. In this case, myocardial ischemia may in the first stages be accompanied by a weakening of the heart muscle, or this occurs against the background of frequent attacks of angina, especially unstable form, Prinzmetal.
Myocardial infarctions, in particular repeated, extensive, atypical ones, lead to the formation of this condition. In such cases, such an outcome is a protective mechanism for the survival of patients in vascular accidents.
Risk factors for ischemic cardiomyopathy include:
- complicated heredity;
- male gender;
- arterial hypertension;
- increased cholesterol levels in the blood;
- abuse of nicotine, alcohol or drugs;
- excess body weight;
- deposition of amyloid protein in the myocardium;
- kidney failure;
- diabetes.
For women, the risk of cardiomyopathy increases after menopause or taking contraceptive pills, as well as in the presence of one or more additional causes.
We recommend reading the article about dilated cardiomyopathy. From it you will learn about the forms of the disease and the reasons for its development, characteristic symptoms, diagnostics and treatment methods.
And here is more information about thyrotoxicosis and the heart.
“Falling asleep” of the myocardium in pathology
To describe the processes occurring in the heart muscle, the term hibernation (falling asleep) was proposed. This most fully reflects a reversible decline in function.
In this case, in proportion to the decrease in blood supply to the myocardium, its need for nutrients decreases.
This process prevents necrosis (infarction) from developing, the cells remain viable, and after the restoration of blood flow they self-heal.
In addition to the generating myocardium, the following processes develop in the heart muscle:
- decreased contractility of muscle fibers;
- stretching of ischemic areas in the systole phase;
- expansion of cavities;
- partial hypertrophy;
- fibrous (sclerotic) changes;
- apoptosis of cardiomyocytes – programmed breakdown into separate fragments.
Myocardial hypertrophy in cardiomyopathy
Symptoms of ischemic dilated cardiomyopathy
In the initial stages, signs of cardiomyopathy may be completely absent. The clinical picture of the disease corresponds to angina pectoris - attacks of pressing pain behind the sternum that occur after physical or emotional stress, and then at rest.
As myocardial damage spreads, its contractility weakens. This is especially true for the left ventricle. Therefore, at later stages, the signs of the disease do not differ from the characteristic manifestations of circulatory failure:
- labored breathing,
- accelerated pulse,
- swelling in the legs,
- congestive wheezing in the lungs,
- cough,
- increased weakness.
A distinctive feature of ischemic cardiomyopathy is the rapid progression of symptoms. Compared with dilated idiopathic cardiomyopathy, thromboembolism and arrhythmia are less common with myocardial ischemia.
Watch the video about cardiomyopathy and its consequences:
Treatment of the patient
There is no specific treatment for cardiomyopathy, so symptomatic therapy and prevention of complications are carried out. The following tools are used for this:
- nitrates for attacks of heart pain - Cardiket, Monosan;
- non-selective adrenergic receptor blockers – Carvedilol;
- calcium antagonists – Corinfar, Diltiazem;
- diuretics – Veroshpiron, Lasix;
- cardiac glycosides - Strophanthin, Digoxin for tachycardia;
- antiarrhythmic - Cordarone;
- anticoagulants – Aspirin, Plavix;
- hypotensive - Hartil, Zocardis;
- hypolipidemic - Liprimar, Traykor;
- to strengthen the myocardium - Riboxin, Mildronate, Neoton, Coraxan.
In this case, it is necessary to follow a dietary diet with a limitation of table salt and liquid, animal fats and sugar, and flour products. Physical activity for each patient is calculated individually, it is necessary to avoid overwork and stress.
When indicated, heart surgery is performed:
- installation of a pacemaker (with or without a defibrillator);
- vascular bypass;
- balloon expansion with stenting;
- removal of atherosclerotic plaque;
- heart transplant.
Death as a real threat with ICMP
Often, the progression of heart failure cannot be stopped, despite complex therapy. This increases the risk of death from the following complications:
- pulmonary edema,
- cardiogenic shock,
- sudden cardiac arrest;
- recurrent myocardial infarction,
- ventricular fibrillation,
- pulmonary embolism.
Ventricular fibrillation as a complication of ischemic cardiomyopathy
Once diagnosed, only a third of patients have a 5-year chance of survival.
This is possible with complete cessation of bad habits, control of blood pressure, cholesterol and glucose levels in the blood, maintenance of normal body weight and adequate therapy.
With early treatment or after transplantation, the prognosis is more favorable. There are cases of 10-year survival after heart transplantation.
We recommend reading the article about restrictive cardiomyopathy. From it you will learn about the pathogenesis and forms of the disease, symptoms, methods of diagnosis and treatment.
And here is more information about the forms of atrial fibrillation.
Ischemic cardiomyopathy develops as a consequence of insufficient coronary blood flow. Some myocardial cells enter a dormant state, the strength of heart contractions decreases, and the cavities expand (especially the left ventricle). Progressive heart failure can be fatal if not treated sufficiently.
Symptomatic therapy is recommended; in many cases, a heart transplant is the only chance to save a life.
Source: //CardioBook.ru/ishemicheskaya-kardiomiopatiya/
Diet
With coronary heart disease, the patient must completely reconsider his lifestyle. Giving up bad habits is the main component of treatment. The diet of a patient with ischemic cardiomyopathy should be based on a balanced diet and moderate limitation of foods containing cholesterol, salt and animal fats. It is necessary to give preference to foods rich in vitamins, especially products that contain ascorbic acid.
Fish and meat should only be consumed boiled. But fish and meat broths should be excluded.
It is also important to reduce the consumption of simple carbohydrates, which are found in foods such as:
- honey;
- sugar;
- confectionery;
- baking;
- rice;
- jam;
- semolina.
Instead, you need to eat complex carbohydrates: non-sweet fruits, grains, vegetables.
We should not forget that frequent stressful situations, nervous strain and limitation of physical activity contribute to the development of coronary disease.
Treatment of cardiomyopathy in a pregnant woman
If the systemic and placental circulation is disrupted, pregnancy most often occurs with complications. Therefore, patients require constant medical supervision in a hospital setting. There, the condition of the expectant mother and fetus is monitored; in case of critical hemodynamic disturbances and a threat to life, emergency delivery is offered.
There is no specific treatment for cardiomyopathies. Therapy is aimed at eliminating the consequences of heart failure, rhythm disturbances and preventing thromboembolic complications. For this purpose they prescribe:
- diuretics in low doses - Hypothiazide, Lasix;
- cardiac glycosides for tachyarrhythmias and low cardiac output - Digoxin, Strophanthin;
- beta blockers – Carvedilol, Metoprolol;
- calcium antagonists – Isoptin, Diltiazem;
- anticoagulants - Heparin, Clexane.
In peripartum cardiomyopathy, good results were obtained from the use of Parlodel.
To limit the load on the heart muscle, gentle or bed rest is recommended, reducing the salt content in the diet to 2 - 3 g, free fluid to 1 liter.
The diet should be dominated by plant foods, boiled fish, chicken, turkey, and fermented milk drinks.
Any salty, fatty, spicy, canned foods, industrial sauces, and semi-finished products should be completely excluded. You need to take food in fractional quantities, avoid any stimulants - coffee, strong tea, tonic drinks.