Edwards syndrome is a form of a rare genetic disorder in which part of a person's 18th chromosome is duplicated. Most children with this pathology die at the stage of embryonic development; this happens in 60% of cases. The prevalence of Edwards syndrome averages 1:3000–1:8000 cases. Inheritance of the syndrome is not traceable, and the randomness of this mutation is only 1%.
Edwards syndrome was named after Dr. John Edward, who described the first cases in 1960 and documented the pattern of symptoms. Edwards syndrome affects more females than males—about 80 percent of those affected are women. Women over thirty years of age have a greater risk of having a child with the syndrome, although the same can happen to women under thirty, but much less frequently. About 12% of children with the syndrome survive to the age when mental development can be assessed; the babies who survive are severely handicapped and usually do not live long. Edwards syndrome is associated with a wide range of disorders that consist of more than one hundred and thirty discrete defects related to the brain, heart, craniofacial structure, kidneys, and stomach.
Causes of development of Edwards syndrome.
Each cell in the human body contains twenty-three pairs of chromosomes, which it inherits from its parents. In humans, each sex cell contains the same number of chromosome sets. In women these are eggs (called XX), and in men (called XY) these are sperm. During the division of a fertilized egg, a mutation occurs under the influence of some factors and an additional chromosome appears in the eighteenth pair, which is responsible for the occurrence of Edwards syndrome. Children with the syndrome inherit the wrong number of chromosomes; instead of two copies, they have three copies of chromosomes. This type of mutation is called “trisomy”; the name is also accompanied by the number of the pair in which the mutation occurred; in the case of Edwards syndrome, this is the eighteenth pair.
The variant described above is a “complete trisomy”, i.e. the child has inherited a complete additional copy of an extra chromosome; statistically, 95% of children with Edwards syndrome are like this. “Full trisomy” has almost all the signs of the disease and is very difficult.
There are two more variants of mutations. Two percent of children with Edwards syndrome have a translocation in pair 18, where only part of the extra chromosome is present. Three percent of children with the syndrome have “mosaic trisomy,” this type of mutation is characterized by the presence of an extra chromosome not in all cells of the body.
Causes
As mentioned above, the disease is genetic; it is based on a mutation - the appearance of a third (additional) 18th chromosome. As a result, the genetic set contains not 46, but 47 chromosomes.
The causes of the genetic mutation are not clear. It is believed that the risk factors are the following: the age of the mother, her bad habits and the presence of chronic diseases, poor living and working conditions (constant exposure to adverse environmental factors). The family history of the child’s mother is also important.
Symptoms of Edwards syndrome.
Most children born with Edwards syndrome are underweight and have severe developmental delays. Their head is unusually small, and the back of the head is of pronounced size. Their ears are low set and their upper and/or lower jaws have a developmental defect called micrognathia. This is a condition when the shape of the face is distorted and an incorrect bite is formed.
Appearance of a patient with Edwards syndrome
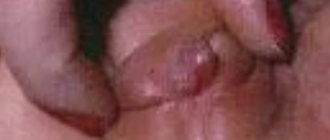
Children often develop cleft palate and cleft lip, conditions where a cleft occurs in the upper palate and lip, respectively.
Formed “cleft lip”
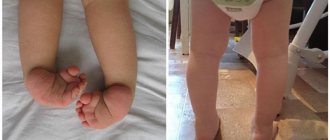
Children's hands are often clenched into fists and all fingers have a characteristic uneven position.
Flexion position of the hand
Children suffer from clubfoot, and the toes on the lower extremities may be webbed or completely fused together.
Very often, patients with the syndrome experience problems with the heart, lungs and diaphragm due to vascular pathology and the development of congenital defects. Heart defects can be of different nature and number: patent ductus arteriosus, ventricular septal defect, patent foramen ovale.
Mutations can cause the development of inguinal and umbilical hernias, abnormalities of the genitourinary system and dysplastic syndrome. The percentage of development of a particular defect:
System damage and defect (sign) - Frequency % Brain skull and face - 100% microgenia - 96.8% low position and/or deformation of the auricles - 95.6% dolichocephaly - 89.8% high palate - 78% cleft palate - 15% microstomia - 71% Musculoskeletal system - 98.1% flexor position of the hands - 91.4% rocking foot - 76.2% cutaneous syndactyly of the feet - 49.5% clubfoot - 34.9% Central nervous system - 20 .4% Cardiovascular system - 90.4% Digestive organs - 54.9% Genitourinary system - 33.5%
Children with Edward syndrome tend to have feeding problems. Problems arise due to disruption of the innate swallowing and sucking reflexes. Poor development of the sucking reflex and uncoordinated swallowing leads to suffocation of the child.
The child may develop gastroesophageal reflux disease. Congenital clefts of the facial skeleton also cause feeding difficulties. They lead to the need to feed the child through a tube or gastrostomy tube. A specialist can show the child's parents how to properly hold their child's head and body. To prevent reflux, the baby's head should be elevated about thirty degrees or more while the baby eats and for an hour or two after eating.
Forms of the syndrome
The type of such an anomaly is primarily influenced by the stage of embryonic development at which the syndrome occurs in the embryo. There are three types in total:
- Full. The most severe type, accounting for 80% of cases. The tripled chromosome appears at the moment when the fetus was just one cell. It follows that the abnormal chromosome set will be transmitted during division to all other cells and will be observed in each of them.
- Mosaic. The name is given because healthy and mutated cells are mixed together like a mosaic. 10% of those affected by Edwards' symptom suffer from this particular form. The signs of the disease are less pronounced here, but still interfere with the normal development of the child. The extra chromosome appears during the phase when the embryo consists of several cells, so only part of the body or a single organ is affected.
- Possible translocation. Here, not only chromosome nondisjunction is observed, but also an overabundance of information generated by translocation rearrangement. It appears both during the maturation of gametes and during the development of the embryo. The deviations here are not pronounced.
Diagnosis of Edward syndrome
The diagnosis of Edwards syndrome can be made by identifying all of the above external symptoms during physical examination. A more accurate diagnosis can be made by performing a "karyotyping" test, which involves taking samples of the child's blood to determine the chromosomal makeup.
Due to the large number of developmental defects and the very low survival rate of children with Edwards syndrome, antenatal diagnostic methods have now been developed. One of the first and most accessible techniques that is carried out in all antenatal clinics is fetal ultrasound. An ultrasound scan in the early stages of pregnancy may reveal malformations of the brain and limbs, as well as the presence of an abundant amount of amniotic fluid, which should alert the doctor. Upon receipt of these results, it is necessary to send the pregnant woman for more detailed and targeted observation in a hospital.
In the hospital, all necessary general clinical tests should be performed and more specific tests should be carried out, such as echography, Doppler, examination of serum blood markers: β-subunit of human chorionic gonadotropin (βCG), α-fetoprotein (AFP), estriol (E3), 17-hydroxy progesterone . To assess the degree of risk of having children with chromosomal abnormalities in all observations, a specially developed computer program PRISCA is used, taking into account the woman’s age, serum markers and gestational age. It is also necessary to perform transabdominal amniocentesis followed by cordocentesis. In the amniotic fluid, in the presence of Edwards syndrome, the following will be determined: AFP, 17-OP, E3, and in the fetal blood the karyotype of trisomy 18 chromosome will be determined.
Diagnostics
Edwards syndrome is an indication for termination of pregnancy, so its presence must be identified as soon as possible. This can be done using prenatal screening - a set of laboratory tests of maternal serum for hormones, including those produced by the fetal membrane:
- beta-hCG;
- PAPP test;
- AFP (alpha fetoprotein);
- free estriol.
You can also suspect the presence of this pathology in a child by performing ultrasound and Dopplerography of the uteroplacental blood flow. Of course, the results of such studies may be indirect and not be the final “verdict”. This applies to both instrumental and laboratory diagnostic methods.
The assessment of the risk of having a child with such deviations is carried out taking into account:
- gestational age;
- age of the expectant mother;
- biochemical and ultrasound screening data;
- body weight of a pregnant woman.
For pregnant women at high risk, doctors suggest undergoing prenatal diagnostics, which includes chorionic villus biopsy, amniocentesis, and cordocentesis. After this, fetal karyotyping is performed.
If Edwards syndrome is detected in a child after birth, it is necessary to immediately conduct a comprehensive examination to identify severe developmental defects. To do this, an examination is carried out by a number of specialists:
- cardiologist;
- neurologist;
- pediatrician;
- surgeon;
- orthopedist;
- urologist.
In the first hours after the birth of a child with a suspected diagnosis of Edwards syndrome, it is important to conduct an echocardiogram, ultrasound of the abdominal organs and kidneys.
Treatment of Edwards syndrome
The scientific medical community does not currently know a cure for the syndrome. Children with Edwards syndrome are usually born with physical abnormalities, and doctors are faced with a challenge regarding treatment options. Surgery can correct some defects associated with the syndrome, but the invasiveness of the technique does not justify the results in children, whose life expectancy ranges from several days to months.
Treatment today comes down to palliative care (maintaining the morale of those facing a fatal disease). Five to ten percent of babies with Edwards syndrome survive their first year of life.
Problems associated with disorders of the nervous system and muscle tone affect the development of the child’s motor skills, which can lead to scoliosis, muscle atrophy, and strabismus. Surgical treatment in sick children may be limited due to malformations of the cardiovascular system. Children with the syndrome often experience constipation caused by poor abdominal wall tone and atonic intestines. As a result, infants experience discomfort and have difficulty feeding. Special milk formulas, laxatives and drugs from the group of “defoamers” can alleviate these symptoms. Enemas are contraindicated in this situation because they can lead to electrolyte imbalance in the child.
Children with the syndrome are often developmentally delayed; therefore, it is necessary to use specially designed therapy programs to correct delayed physical development.
Another factor for a poor prognosis is the increased risk of developing a Wilms tumor, a type of kidney cancer. Regular ultrasound examination of the abdominal cavity for the presence of this pathology is recommended.
Sick children are at risk of developing various infections and pathological conditions, such as: genitourinary system infections, otitis media, conjunctivitis of various etiologies, sinusitis, sinusitis, pneumonia, sleep apnea, pulmonary hypertension, congenital heart defects and high blood pressure. It is necessary to be prepared in order to effectively carry out treatment and suspect these diseases in time.
Parents whose child has this syndrome need constant monitoring of their health, since timely detection of the disease can prolong the life of their children.
What should parents do if Edwards Syndrome is detected in the fetus?
The exact causes of the chromosomal mutation have not yet been identified. There is a risk of children being born with pathologies in an older mother, but in the case of this Syndrome, a sick child can be born to an actually healthy mother and father. The main thing that parents can do is to undergo all examinations at the pregnancy planning stage, and if necessary, get advice from a geneticist.
All symptoms of the disease can be detected by ultrasound, which is performed at the end of the first trimester of pregnancy.
The most difficult part of such a problematic pregnancy is making a decision. Edwards syndrome is a much more severe pathology than Down syndrome. In the latter cases, these children can succeed in life, find themselves, and be socially useful. Edwards syndrome actually leaves no chance for this.
Therefore, when a pathology is detected prenatally:
- Parents in most cases decide to terminate the pregnancy, which in the case of this diagnosis is possible at a later date if the pathology was not detected immediately;
- After termination of pregnancy, parents undergo genetic counseling to identify possible causes of such disorders.
The birth of such sick children in rare cases is a conscious choice of parents. More often these are children of mothers who did not register for pregnancy and did not undergo all possible studies. Usually parents abandon a child who is practically doomed to death.
It is worth knowing that it is almost impossible not to see pathology on an ultrasound. This is why it is so important for a pregnant woman to follow all doctor’s instructions during gestation, take all tests on time, and complete the first screening in full. If, through research, severe pathologies are discovered, termination of pregnancy in the first trimester will not be as painful or psychologically traumatic for the mother as a late abortion or artificial birth. Almost every medical institution today has a psychologist who can be contacted by a woman (or both parents) who find themselves in such a difficult situation.
Edward syndrome prognosis
The prognosis for the most part for this pathology is not favorable. Most children who are born with Edwards syndrome do not survive their first year of life. The average life expectancy for half of children born with this syndrome is less than two months. Ninety to ninety-five percent of these children die before their first birthday. Five to ten percent of children who survive the first year experience severe developmental disabilities.
Children who have lived through their first year require constant monitoring and supervision, and experience serious developmental difficulties. Their verbal communication skills are limited, although they are able to respond to their parents' comfort and have the opportunity to learn to smile, recognize and interact with caregivers and others. They can acquire skills needed for all children, such as self-feeding and head lifting, skills common to healthy children their age.
General practitioner Zhumagaziev E.N.
Causes
Unfortunately, doctors have not yet clarified the reasons for the mutation. It is only known that girls are susceptible to this pathology several times more often than boys, and that mothers over the age of 30 are more likely to have a baby with a disorder such as Edwards syndrome.
The reasons for the appearance of Edwards syndrome in a child do not depend on the health status of the parents - the mother and father may be completely healthy, as well as their immediate relatives, but the child may be born with a pathology. And since the causes cannot be established, it means that it is impossible to prevent the development of the disease in the fetus. It is very important that every pregnant woman registers and is examined in a timely manner, which makes it possible to identify Edwards syndrome in her baby in the early stages and terminate the pregnancy.
Sometimes parents decide to save the life of a sick child - in this case, it is important to understand that if the child does not die in infancy, he will require outside help throughout his life and no treatment will help him.
Note that the mortality rate in the womb of such children is 60%. Among those who are nevertheless born with a deviation, 90% of cases die in the first year of life, and only a few survive to adolescence or older age. That is, the prognosis for the course of such a pathology is extremely unfavorable.
Prenatal diagnosis
Edwards syndrome can be determined on ultrasound only by indirect signs. The most accurate method for diagnosing the syndrome in a fetus today is perinatal screening. Based on this, if alarming suspicions arise, the doctor already refers the woman to invasive testing.
Screening that identifies the karyotype of Edwards syndrome is divided into two stages:
- The first is carried out at 11-13 weeks of pregnancy. Biochemical indicators are examined - the mother's blood is checked for hormone levels. The results at this stage are not final - they can only indicate the presence of risk. For calculations, a specialist needs protein A, hCG, a protein produced by the membranes of the embryo and placenta.
- The second stage is already aimed at an accurate result. For research, a sample of umbilical cord blood or amniotic fluid is taken, which is then subjected to genetic analysis.
Invasive testing
Chromosomes of Edwards syndrome are most likely to be determined by this method. However, it necessarily involves surgical intervention and penetration into the membranes of the embryo. Hence the risk of miscarriage and the development of complications, which is why the test is prescribed only in extreme cases.
Today there are three types of sampling known:
- CVS (chorionic villus biopsy). The main advantage of the method is that a sample is taken starting from the 8th week of pregnancy, which allows complications to be identified in the early stages. For research, you need a sample of the chorion (one of the layers of the placenta membrane), the structure of which is similar to the structure of the embryo. This material allows you to diagnose intrauterine infections, genetic and chromosomal diseases.
- Amniocentesis. The analysis is carried out starting from the 14th week of pregnancy. In this case, the amniotic membranes of the embryo are pierced with a probe, the instrument collects a sample of amniotic fluid containing cells of the unborn child. The risk of complications from such a study is much higher than in the previous case.
- Cordocentesis. The deadline is no earlier than the 20th week. This is where a sample of the embryo's umbilical cord blood is taken. The difficulty is that when taking the material, the specialist has no room for error - he must hit the needle exactly into the umbilical cord vessel. In practice, this happens like this: a puncture needle is inserted through the anterior wall of the woman’s peritoneum, which collects about 5 ml of blood. The procedure is carried out under the control of ultrasound devices.
All the methods described above cannot be called painless and safe. Therefore, they are carried out only in cases where the risk of a genetic disease in the fetus is higher than the risk of complications from taking material for analysis.
Parents need to remember that a doctor’s mistake during the procedure can lead to serious diseases and congenital defects in the unborn child. The risk of sudden termination of pregnancy from such an intervention cannot be excluded.