Abdominal aortic aneurysms (AAA) and their complications occupy a prominent place in the structure of morbidity in the cardiovascular system. Rupture of an AAA has the most severe course and poor prognosis. In recent years, there has been a pronounced tendency to increase the number of such patients. A significant part of them are admitted to general surgical hospitals with suspected intra-abdominal catastrophe. Sometimes the real cause of an acute abdomen is revealed only during emergency laparotomy. Without exaggeration, the fate of patients depends on how quickly surgeons can correctly navigate a difficult clinical situation and undertake adequate surgical intervention. If such a patient is denied surgical treatment, death is inevitable. Unfortunately, a vascular surgeon is not always able to come to the rescue. That is why we believe that doctors providing emergency care to patients with abdominal surgical pathology should be informed about the methods of diagnosing and treating AAA.
Occurrence of disease Rupture of abdominal aortic aneurysm
It is known that aortic aneurysms are divided into congenital (in Marfan disease) and acquired. The latter, depending on their genesis, are divided into: non-inflammatory (atherosclerotic, traumatic) and inflammatory (syphilitic, mycotic, due to nonspecific aortitis). More than 95 % of abdominal aortic aneurysms are of atherosclerotic origin.
Based on their shape, it is customary to distinguish fusiform and saccular aneurysms. In the first case, there is a diffuse expansion of the aorta, when its entire circumference is involved in the process. A saccular aneurysm is a protrusion of one of the walls of the aorta with a more or less narrow channel communicating with it - the “neck”. Fusiform aneurysm develops more often than saccular aneurysm. The latter communicates with the aorta over a limited extent and often contains a large amount of thrombotic masses, so the wall of such an aneurysm is less susceptible to intra-aortic pressure.
The size of the aneurysm is the most important indicator, since the extent of the aneurysm is the main factor determining the extent of surgical intervention. The classification of A.A. fully meets practical purposes. Pokrovsky et al. (1975), providing for 4 types of abdominal aortic aneurysms.
Type I - aneurysm of the proximal segment of the abdominal aorta involving the visceral branches;
Type II - aneurysm of the infrarenal segment before the bifurcation;
Type III - aneurysm of the infrarenal segment involving the bifurcation of the aorta and iliac arteries;
Type IV - total damage to the aorta.
The diameter of the aneurysm, as well as its type, are not so significant for the surgeon. The fact that the diameter of the aneurysm influences its tendency to rupture deserves attention. Aneurysms of large diameter rupture more often, since the pressure on the wall is directly proportional to the radius of the aneurysmal sac and, therefore, as the diameter of the aorta increases, the “rupture” force acting on its walls increases.
The direction (localization) of the rupture is important, since it determines the anatomical area into which the blood is released (free abdominal cavity, retroperitoneal space, intestine, inferior vena cava, etc.). Experience shows that the left lateral wall of the aneurysm is most often subject to rupture, which determines the highest frequency of retroperitoneal rupture of abdominal aortic aneurysms.
Various types of ruptures of abdominal aortic aneurysms are known - from the most common (in the retroperitoneal space) to the casuistically rare (bladder). Since each variety differs in clinical features and prognosis, we use the following classification:
1. Rupture into the retroperitoneal space;
2. Rupture into the free abdominal cavity;
3. Rare forms of rupture of an abdominal aortic aneurysm (into the inferior vena cava, duodenum, etc.)
Causes and risk factors
The results of numerous studies have shown that the main etiological factor of an abdominal aortic aneurysm, as well as other localizations of this pathological process (thoracic aorta, aortic arch), is atherosclerosis. In 80-90% of cases, the development of the disease is caused by it. Much less frequently, the development of acquired abdominal aortic aneurysms is associated with inflammatory processes (rheumatism, mycoplasmosis, salmonellosis, tuberculosis, syphilis, nonspecific aortoarteritis).
Often, an abdominal aortic aneurysm forms in patients with congenital defects in the structure of the vascular wall (fibromuscular dysplasia).
Causes of traumatic aneurysm of the abdominal aorta:
- spinal and abdominal injuries;
- technical errors when performing reconstructive operations (prosthetics, thromboembolectomy, stenting or aortic dilatation) or angiography.
Factors that increase the risk of developing an abdominal aortic aneurysm are:
- smoking – smokers make up 75% of all patients with this pathology; the longer the smoking history and the number of cigarettes smoked daily, the higher the risk of developing an aneurysm;
- age over 60 years;
- male gender;
- the presence of this disease in close relatives (hereditary predisposition).
Rupture of an abdominal aortic aneurysm most often occurs in patients suffering from chronic bronchopulmonary diseases and/or arterial hypertension. In addition, the size and shape of the aneurysm influence the risk of rupture. Symmetrical aneurysmal sacs rupture less frequently than asymmetrical ones. And giant dilations, reaching 9 cm in diameter or more, rupture in 75% of cases with massive bleeding and rapid death of patients.
Symptoms of the disease Rupture of an abdominal aortic aneurysm
Classification
Since there are certain differences in the published classifications of abdominal aortic aneurysms complicated by rupture, we chose the most practical one based on a number of characteristics and made some adjustments to it taking into account its use in patients with ruptured AAA. The division is based on: the etiology of the aneurysm, its shape, the location of the rupture and its type, the amount of blood loss.
Classification of abdominal aortic aneurysms
By etiology: congenital and acquired (atherosclerotic, traumatic, syphilitic, mycotic, due to nonspecific aortitis)
Shape: spindle-shaped, bag-shaped
By type (prevalence) of damage:
Type I - damage to the proximal segment of the abdominal aorta with involvement of the visceral branches;
Type II - damage to the infrarenal segment before the bifurcation;
III - infrarenal segment involving the bifurcation and iliac arteries;
IV - total damage to the aorta.
Localization (direction) of the rupture: retroperitoneal space, free abdominal cavity, rare (inferior vena cava, duodenum, bladder, etc.).
Rupture of an abdominal aortic aneurysm occupies a special place among other causes of “acute abdomen” not only because of the catastrophic course and the inevitability of death in a relatively short period of time without emergency surgery. The fact is that an error in diagnosing other acute diseases of the abdominal organs (for example, if instead of acute appendicitis a perforated ulcer is discovered during surgery) does not lead to significant difficulties in performing the intervention. If, during laparotomy, a ruptured abdominal aortic aneurysm is accidentally discovered, the surgeon may find himself in an extremely difficult position: he must have sufficient experience in vascular surgery.
The question of whether diagnosing a ruptured AAA is simple or difficult cannot be given a definite answer. On the one hand, in the presence of a characteristic triad of signs: pain in the abdomen and lumbar region, collapse, pulsating formation in the abdominal cavity, the diagnosis is really not difficult. On the other hand, there would not be a large number of diagnostic errors (50-70% of cases) if the recognition of a ruptured aneurysm was so simple.
What are the specific tasks facing a clinician when examining a patient suspected of having this disease? They can be formulated as follows. First, it is necessary to establish that the patient actually has an abdominal aortic aneurysm. Experience shows that there is often overdiagnosis of an aneurysm due to a tortuous, atherosclerotically altered and intensely pulsating abdominal aorta, which is especially palpable in thin people suffering from hypertension. At the same time, in obese people an aneurysm of even significant size may not be palpable. Secondly, it is required to prove the fact of aneurysm rupture. In practice, there are cases when abdominal pain in a patient with an abdominal aortic aneurysm is explained not by its rupture, but by other reasons. This may lead to an erroneous diagnosis of a ruptured AAA and emergency intervention, instead of operating on the patient as planned after appropriate examination and careful preparation. On the other hand, in some patients with a diagnosed AAA, signs of its rupture may be so mild that they escape the doctor’s attention.
The clinical picture of a ruptured abdominal aortic aneurysm is quite diverse, which is primarily determined by the type of rupture.
With retroperitoneal rupture of an abdominal aortic aneurysm, the pain syndrome is permanent. Most often, pain occurs in the abdomen or lumbar region. The nature of the pain is associated with the location and extent of the retroperitoneal hematoma. With extensive, intense hematomas, compression of the nerve trunks and plexuses occurs, which causes a particularly painful and persistent pain syndrome.
The irradiation of pain also has a direct connection with the localization and extent of retroperitoneal hematoma. So, if the lower pole of the hematoma reaches the pelvis, irradiation of pain is observed in the groin area, thigh, and perineum. With a high spread of hematoma, pain radiates upward, more often to the heart area.
It is necessary to note one important circumstance, which is the following. In most patients, the severity of the pain syndrome does not correspond to the objective symptoms of the abdomen, which manifest themselves very moderately. The latter are caused by stretching of the parietal peritoneum and extravasation of blood into the abdominal cavity. It should be emphasized that the amount of blood in the free abdominal cavity during retroperitoneal rupture of an aneurysm is usually small - no more than 200 ml. Apparently, this explains the absence of pronounced peritoneal symptoms. Detection of a pulsating formation in the abdominal cavity is extremely important for diagnosing an aneurysm . This succeeds in approximately 70-80% of cases. A non-pulsating formation may also be detected, which is explained by massive thrombosis of the aneurysm cavity or a large retroperitoneal hematoma.
The bleeding syndrome, if expressed, plays a decisive role in establishing the clinical diagnosis of ruptured AAA . The most acute manifestations of this syndrome (collapse with loss of consciousness) occur in approximately 20% of patients. In the rest, the clinical manifestations of internal bleeding are very moderate. This is characteristic of a retroperitoneal rupture, since the outpouring of blood into the retroperitoneal space occurs relatively slowly, which leaves time for the activation of compensatory hemodynamic mechanisms. This is where difficulties arise in diagnosing retroperitoneal AAA rupture, since it is reasonable to expect more significant hemodynamic disorders with such a formidable disease. That is why the weak severity of blood loss syndrome or its absence should not serve as a basis for denying the diagnosis of ruptured AAA . This feature of the clinical picture of the disease should be remembered, and then there will be fewer unjustified delays in hospitalization of patients and unnecessarily long observation of them in a hospital setting.
Intraperitoneal rupture of an abdominal aortic aneurysm is characterized by extremely severe clinical manifestations: a rapid increase in symptoms of internal bleeding and hemorrhagic shock. When examining the patient, a sharp pallor of the skin, covered with cold sweat, is noted. The pulse is frequent, thread-like. The abdomen is swollen, sharply painful in all parts. A diffuse Shchetkin-Blumberg symptom is determined. Percussion of the abdomen reveals free fluid in the abdominal cavity. The catastrophic course of the disease in such cases precludes any diagnostic measures. Death occurs very quickly.
When an AAA ruptures (breaks through) into the inferior vena cava, patients complain of weakness, shortness of breath, and palpitations. Local symptoms are characterized by pain in the abdomen and lumbar region, the presence of a pulsating formation in the abdomen with a characteristic systole-diastolic murmur above it. Swelling of the lower extremities and lower half of the body is noted. These symptoms develop gradually but progress steadily, leading to severe heart failure.
Rupture of an abdominal aortic aneurysm into the duodenum is characterized by symptoms of profuse gastrointestinal bleeding: sudden collapse, hematemesis, melena. This symptom complex is difficult to differentiate from bleeding into the gastrointestinal tract of another etiology. The diagnosis is facilitated in cases where there is anamnestic indication of an abdominal aortic aneurysm or it is determined by palpation of the abdomen.
Establishing the type of aneurysm based on clinical data is a complex and not always solvable task. In this regard, the most practically important thing is to determine the upper pole of the AAA, i.e. its relationship with the renal arteries. De Baci's symptom is of significant importance, namely: the ability to “bypass” its upper pole with the hand when palpating the aneurysm. If this is successful (i.e., De Bakey's symptom is positive), then the proximal border of the aneurysmal dilatation of the aorta is located below the mouth of the renal arteries. Unfortunately, this symptom, which provides reliable information in the case of a chronic aneurysm, is not so informative when it ruptures. First of all, this is due to the presence of a para-aortic hematoma, masking the true boundaries of the aortic lesion. It follows that a negative De Baca symptom does not yet mean that the expansion of the aorta has spread to the level of the renal arteries and above.
Epidemiology
Sign of prevalence: Extremely rare
Sex ratio(m/f): 5
An abdominal aortic aneurysm is found, according to various authors, in 0.16-1.06% of all autopsies. The male to female ratio is 5:1. With increasing age, the frequency of the disease increases sharply - for men who died before the age of 50, the frequency of abdominal aortic aneurysms is 6%, over 60 years - 10%, over 70 years - 12%. Among aortic aneurysms, aneurysms of the abdominal aorta make up the majority—80%. In 95-96% of patients, aneurysms are usually located below the renal arteries. There is also a direct relationship between the size of aneurysms and their tendency to rupture. For small aneurysms (aortic diameter up to 5 cm), survival rate within 1 year is 75%, within 5 years - 48%. If the diameter of the aneurysm is more than 6 cm, then the survival rate within a year is 50%, within 5 years - only 6%.
Diagnosis of the disease Rupture of abdominal aortic aneurysm
Diagnostic errors
Analysis of our observations and literature data show that AAA rupture represents a complex diagnostic problem. The range of diagnostic errors is very wide. The category of patients with ruptured AAA who are mistakenly diagnosed with any disease of the “acute abdomen” group is the largest. The most common erroneous diagnoses are: acute cholecystitis, pancreatic necrosis, destructive appendicitis, etc. Pain in the lumbar region, which is observed in most patients with ruptured AAA, may suggest an acute urological disease. Acute pain in the lower back, accompanied by radicular syndrome with irradiation of pain to the legs, in some cases serves as the basis for a diagnosis of sciatica. Finally, some patients are admitted to the clinic with a diagnosis of acute obstruction of the aortic bifurcation and arteries of the lower extremities. Actually, this would not be a completely erroneous diagnosis, since in each of these cases, AAA rupture was combined with acute thrombosis or embolism of the underlying arterial lines. The essence of the error was that the attention of doctors was focused only on the clinical signs of ischemia of the lower extremities, and a more important circumstance - rupture of the AAA - was overlooked. Thus, diagnostic errors significantly affect the fate of patients. Knowledge of the main clinical manifestations of this disease and a thorough physical examination of patients allows us to make a correct diagnosis or at least suspect a ruptured aneurysm. At the same time, it is not uncommon for such cases when a differential diagnosis of AAA rupture with other clinically similar diseases is beyond the capabilities of even an experienced clinician. In this case, special research methods provide invaluable assistance.
Instrumental diagnostics
A non-invasive research method - ultrasound angioscanning - has good information content. The use of this method does not worsen the condition of patients and provides reliable information about the size of the aneurysm, the location of the rupture and the size of the para-aortic hematoma.
CT scan. In recent years, preoperative spiral computed tomography has been successfully used, which makes it possible to accurately determine the location and size of the aneurysm, its relationship to the renal arteries and other branches of the abdominal aorta, and accurately determine the location of the rupture. And when stenting the aorta, CT helps determine the required size of the stent. Signs indicating a ruptured AAA are:
1. Detection of para-aortic hematoma; Moreover, it is possible to differentiate between “fresh” and “old” hematomas.
2. Displacement of retroperitoneal organs by hematoma.
There is no doubt that CT is a highly informative and safe diagnostic method.
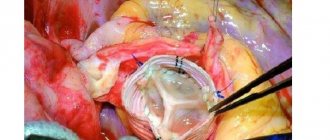
Aortography. A more traumatic, but in some patients absolutely necessary, research method is aortography (Fig. 17.1). It is indicated in clinically unclear cases in order to establish a definitive diagnosis. But even with an established diagnosis of AAA rupture, X-ray contrast examination provides valuable additional information: 1) determining the relationship of the aneurysm with the visceral branches of the abdominal aorta, its spread to the aortic bifurcation and iliac arteries; 2) clarification of the nature of occlusive lesions of the visceral arteries and main arteries of the extremities; 3) identification of an aortocaval fistula (Fig. 17.2). It should be noted that with aortography it is not always possible to confirm the fact of aneurysm rupture, since extravasation of the contrast agent may not occur due to tamponade of the defect in the wall of the aneurysmal sac with blood clots. A contraindication to aortography is the extreme severity of the patient’s condition and unstable hemodynamics with a decrease in blood pressure below 90 mmHg.
In some cases, laparoscopy provides significant assistance in the differential diagnosis of AAA rupture and acute diseases of the abdominal organs. Detection of free blood in the abdominal cavity, hematomas in the retroperitoneal tissue, mesentery of the small and large intestines makes the diagnosis sufficiently definite. However, these signs are not strictly specific to aneurysm rupture, as they can be observed with hemorrhagic pancreatic necrosis, as well as with traumatic injuries of the abdominal organs and retroperitoneal space.
Laparoscopy is indicated in patients with suspected AAA rupture provided: 1) stable hemodynamics (with systolic blood pressure not lower than 90 mm Hg), 2) it is impossible to perform ultrasound as the primary method of instrumental examination.
Endoscopic signs of rupture of an abdominal aortic aneurysm are divided into direct and indirect.
Direct signs of a ruptured AAA include:
1. Hematoma of the retroperitoneal tissue and mesentery of the small intestine.
2. Liquid blood or intensely colored serous fluid in the abdominal cavity.
This method is of particular importance in the postoperative period (dynamic laparoscopy).
Prevention
For timely detection of abdominal aortic aneurysm, patients suffering from atherosclerosis or having a history of this vascular pathology are recommended to undergo systematic medical observation with periodic instrumental examination (radiography of the abdominal cavity, ultrasound).
Quitting smoking and active treatment of infectious and systemic inflammatory diseases are of no small importance in the prevention of aneurysm formation.
Video from YouTube on the topic of the article:
Version: MedElement Disease Directory
Treatment of the disease Rupture of abdominal aortic aneurysm
Emergency surgical interventions in patients with ruptured AAA are a complex problem. They are accompanied by high mortality and a large number of complications. At the same time, refusal to operate is tantamount to imposing a death sentence on the patient.
Based on the above, there is no alternative to surgical intervention for a ruptured aneurysm. However, surgical activity for this pathology cannot be expanded indefinitely, since in some cases the operation is obviously doomed to failure. In this regard, the surgeon must carefully assess the situation so that, on the one hand, not to perform a wasted operation, and on the other, not to deprive the patient of his last chance for salvation.
Five risk factors associated with mortality should be kept in mind: age over 76 years, signs of myocardial ischemia, hemoglobin < 90 g/l, creatinine > 190 mmol/l and lack of consciousness on admission to hospital.
Naturally, an abdominal aortic aneurysm is never an isolated manifestation of general atherosclerosis. Almost every patient has coronary heart disease, and many have atherosclerotic damage to the cerebral vessels. All this significantly aggravates the condition of patients, especially if there is a history of myocardial infarction or cerebrovascular accident. However, these circumstances do not make the forecast completely hopeless. However, the presence of a recent myocardial infarction or acute cerebrovascular accident in a patient completely excludes the possibility of surgical treatment of a ruptured AAA.
Impaired urinary function in this group of patients is a poor prognostic sign, since even with normal initial diuresis after aneurysmectomy, progressive renal failure often develops, which in many cases causes death. The fight for the life of a patient with a ruptured AAA should begin in the hospital emergency room. Ideally, catheters should be installed in the central vein, in the bladder, and the main indicators of hemostasis, blood type, Rh factor, etc. should be determined.
It is advisable to hospitalize the patient in the intensive care unit, where, along with ongoing preparation for the operation, ultrasound and, if possible, CT scans are performed. In addition, cardiac, pulmonary and renal function should be assessed. In cases where immediate surgery is not possible in some cases, there are bloodless methods to temporarily stop bleeding from a ruptured aortic aneurysm. Pneumatic body compression has been successfully used to stop bleeding from the aorta for a period of about 2-5 hours. However, the scope of its use is limited by the time of transportation of the patient.
Surgery for a ruptured abdominal aortic aneurysm belongs to the category of surgical interventions in which speed and a clear sequence of manipulations are of particular importance. The slightest mistake or deviation from the optimal technical version of the operation for a given situation can cost the patient his life. This is why it is necessary to elaborate on the technical details of the intervention.
The optimal surgical approach for ruptured abdominal aortic aneurysm is a median laparotomy from the xiphoid process of the sternum to the pubis. This surgical approach gives wide exposure of the infrarenal aorta and iliac arteries. Some surgeons use transverse laparotomy, making a horizontal incision in the anterior abdominal wall 3-4 cm above the navel. However, this access limits the surgical area, since under conditions of transverse laparotomy, exposure of the iliac arteries is achieved with great difficulty. In addition, the transverse approach is more traumatic and time-consuming compared to the vertical median one. As for thoracophrenolumbotomy, which allows extensive mobilization of the aorta along with the renal arteries and visceral branches, this surgical approach is simply not necessary for intervention for the rupture of an infrarenal aneurysm. In some cases, when the lesion is extensive and when revascularization of the lower extremities is necessary, a combined approach is required: midline laparotomy and femoral access (unilateral or bilateral).
After opening the abdominal cavity, the initial task of the surgeon is to achieve proximal control of the abdominal aorta, i.e. interruption of blood flow in the aorta above the aneurysm. There are three levels of proximal control of the abdominal aorta: infrarenal, suprarenal, subphrenic.
The upper pole of an abdominal aortic aneurysm is most often located 2 cm or more below the origin of the renal arteries. In this regard, one should strive to implement proximal control at the infrarenal level. This critical stage of the operation is performed as follows. After opening the abdominal cavity, the small intestine is moved to the right half of the wound or removed from the abdominal cavity altogether, wrapped in a damp towel. Next, the posterior parietal peritoneum at the root of the mesentery of the transverse colon, the posterior layer of the mesentery and the ligament of Treitz are dissected. The duodenum is shifted to the right, after which the infrarenal portion of the aorta is isolated with a finger. In most cases, this can be done relatively easily, because the tissues surrounding the aorta are displaced to the side by the para-aortic hematoma. Following this, a clamp is applied to the selected area.
In some cases, the left renal vein is a significant obstacle to applying an infrarenal clamp. When visual control is difficult in the setting of a para-aortic hematoma, it can be damaged, resulting in an additional source of bleeding. You should not immediately try to find a defect in the venous wall and sew it up, because this wastes valuable time. It is necessary to perform hemostasis with tamponade of the bleeding area and continue manipulations on the aorta. If the left renal vein proves to be an insurmountable obstacle to applying an infrarenal clamp to the aorta, it can be divided between the clamps near the orifice to preserve functioning collaterals at the renal hilum. After completion of the aortic surgery, the integrity of the transected (or accidentally damaged) renal vein must be restored. In principle, as a last resort, ligation of the renal vein is also acceptable, but this is an additional factor that can aggravate the already frequently developing renal failure in operated patients.
If difficulties arise with isolating the infrarenal aorta, you can use endovascular occlusion of the “neck” of the aneurysm using a Foley catheter or a Fogarty-type obturator balloon.
It should be said that in cases of profuse bleeding from a ruptured aneurysm, endovascular proximal control can be a life-saving measure. The method is not without its drawbacks. Complete obturation of the aorta is not always achieved, which causes leakage of blood in addition to the balloon. It is difficult to determine exactly where the balloon is located: below, at the level of, or above the renal arteries. If there is a sudden increase in blood pressure, the obturator may be displaced downward by the blood stream and cease to perform its function. Finally, balloon obturation, like external compression, is a temporary measure for emergency hemostasis, after which a clamp should be applied to the infrarenal aorta.
Significant difficulties for both proximal control and superior anastomosis arise if the upper pole of the aneurysm is located close to the renal arteries. In this case, it is permissible to apply a clamp at the suprarenal level. The duration of suprarenal aortic clamping should be reduced as much as possible, therefore, immediately after the proximal anastomosis of the aorta with the prosthesis, the clamp should be moved below the renal arteries.
The subphrenic level of proximal control of the abdominal aorta is indicated in cases where: the patient’s condition is so critical that there is no time left to isolate the “neck” of the aneurysm; There is an extensive retroperitoneal hematoma, making it difficult to find and isolate the infrarenal aorta.
The technique of subdiaphragmatic compression of the aorta is as follows. Immediately after opening the abdominal cavity, the left triangular ligament of the liver is dissected and its left lobe is retracted to the right. The cavity of the lesser omentum is opened, the aorta is bluntly isolated immediately below the hole in the diaphragm and compressed with fingers or a clamp (Fig. 17.4). Subphrenic clamping of the aorta is accompanied by ischemia of the kidneys and all abdominal organs, and therefore it should be short-term. It is necessary only for the period of infrarenal (or suprarenal) control, after which it must be immediately eliminated.
These are the methods and levels of proximal control of the abdominal aorta. Each of them has its own advantages and disadvantages, as well as indications for use. More than three decades have passed since Ch. Dubost (1951) performed the first operation for an aneurysm of the abdominal aorta. Over the past period of time, the surgical technique has undergone significant changes, and therefore the operation no longer fully corresponds to the term “aneurysmectomy.” It is now generally accepted that the entire aneurysmal sac should not be removed because this is fraught with injury to organs fused to the aneurysm, increases blood loss and duration of the operation, and results in severe postoperative intestinal paresis. In the modern version, the scope of the operation includes removal of the contents of the aneurysm (atheromatous and thrombotic masses), excision of the anterior and partially lateral walls of the aneurysmal sac, and therefore it would be more correct to call such an intervention “subtotal aneurysmectomy.” Total removal of the aneurysm is permissible if it is small, but this possibility is rare, since large aneurysms usually rupture.
When starting an aneurysmectomy, it is necessary to simultaneously decide which prosthesis to prefer: direct or bifurcation. Unlike a planned operation, the scope of which often includes reconstruction of the renal and other visceral vessels, as well as arteries of the lower extremities, with emergency aneurysmectomy the main task is to save the patient’s life. In this regard, the scope of intervention should, if possible, not be expanded. In particular, direct aortic replacement (tubular prosthesis) is preferable to bifurcation. Unfortunately, the possibility of direct prosthetics is not always possible.
As noted above, in order to reduce the time of the operation and reduce its morbidity, it is not necessary to completely cross the aorta in the area of the “neck” of the aneurysm. The anterior wall of the aneurysm is cut through a vertical incision, which has a T-shape at the proximal end. If direct replacement of the aorta is intended, the lower end of the incision is given the same shape. If the aneurysm involves both common iliac arteries, the incision is made in such a way that it becomes possible to sew a bifurcation graft into the slightly altered areas of the iliac vessels. After the aneurysm cavity is freed from thrombotic and atheromatous masses, the orifices of the lumbar arteries are sutured for final hemostasis. Next, they begin to apply a proximal anastomosis of the aorta with the prosthesis. This is the technically most difficult stage of prosthetics, especially if the stump of the infrarenal aorta is short.
The anastomosis is performed with a continuous enveloping suture. It is necessary to note the danger of cutting through the sutures of the posterior semicircle of the anastomosis. There is no free edge of the aorta and therefore the needle must be inserted to a depth twice the thickness of the aortic wall. In the event that the seam has cut through, it is advisable to apply several U-shaped sutures with synthetic “gaskets” that reliably fix the prosthesis to the aortic wall. If necessary, a blanket suture can be placed between the U-shaped seams. A distal anastomosis is performed in a similar manner, which is much easier to perform due to better exposure of the distal aorta. Before completion of the distal anastomosis, by temporarily loosening the infrarenal clamp, the prosthesis is “wetted” with blood, followed by washing out the clots using a syringe.
The moment of removing the clamps from the aorta is very important. In this case, rapid redistribution of blood occurs and metabolic disorders develop, i.e. revascularization syndrome (after a more or less long period of ischemia of the lower half of the body). In order to prevent possible hemodynamic disorders, the clamp from the aorta should be removed slowly under the control of systemic arterial and central venous pressure.
After removing the clamps, it is necessary to ensure the tightness of the anastomoses. If blood leaks from a needle or between sutures, a napkin moistened with alcohol should be pressed to this place, which is most often sufficient to achieve hemostasis. If this technique does not give the expected effect, it is necessary to apply additional U-shaped sutures. Then, in order to isolate the prosthesis from surrounding organs, the remaining sections of the wall of the aneurysmal sac are sutured over it after their partial excision.
The issue of drainage of the abdominal cavity and retroperitoneal space must be resolved depending on the specific situation. If the possibility of capillary bleeding from the aneurysm bed is foreseen, aspiration (non-suction) drainage should be installed; if the surgeon is confident that final hemostasis will be achieved, there is no need for drainage.
It is important to mention one more circumstance. During the period of isolating the aorta and applying clamps to it, embolism of the iliac arteries and arteries of the lower extremities (thrombi, atherosclerotic plaques) may occur. That is why, before completing the distal anastomosis, it is necessary to check the nature of the retrograde blood flow from both iliac arteries by sequentially briefly removing the clamps from them. However, even in this case, if the retrograde blood flow is good, it is advisable to inspect the arteries using a Fogarty catheter. After completing the vascular stage of the operation, it is necessary to examine the patient’s lower extremities to identify signs of ischemia.
Bifurcation aortoiliac replacement is indicated when the common iliac arteries are involved in the aneurysm, as well as when their mouths have pronounced atherosclerotic changes. Just as with direct aortic replacement, before completing distal anastomoses with the iliac arteries, it is necessary to inspect the peripheral arterial bed using a Fogarty catheter.
In case of widespread atherosclerosis of the iliac arteries, aortofemoral bypass surgery is indicated, which, to save time, is advisable to be performed by two teams of surgeons.
Particular attention should be paid to those cases where blood flow in the lower limb is poorly restored or not restored at all. This can be easily verified by the appearance of the limb, as well as by the results of an ultrasound examination. There is no point in expanding the scope of the operation through femoral-popliteal prosthetics and other revascularization methods, because In this category of patients, severe in many respects, such an intervention will be intolerable. Given this circumstance, in case of severe ischemia, including muscle contracture, it is logical to perform primary amputation of the limb.
In recent years, the installation of an endovascular stent has been used as an alternative to traditional surgical treatment for ruptured AAA. Unlike open surgery, this method is much less invasive. According to the limited literature data, stent placement reduces the mortality rate and the number of postoperative complications. However, for successful installation of an endovascular stent graft into an aortic aneurysm, it is necessary that the proximal “neck” of the aorta should not be shorter than 1.5 cm and there should be no pronounced calcification there. Kinking of the aorta and pelvic vessels makes stent placement more difficult.
A wide range of complications during implantation of endovascular stents has been described. According to the literature, distal migration of the stent prosthesis can occur in 10% of cases. Other complications may include stent occlusion of the renal arteries and graft occlusion (19%).
To summarize, we hope that the information presented in this chapter will serve to improve surgical care for patients with a ruptured abdominal aortic aneurysm.
general information
Abdominal aortic aneurysm means:
- any expansion of the diameter of the infrarenal abdominal aorta by 50% compared with the suprarenal;
- any local fusiform dilatation of the aorta with a diameter 0.5 cm larger than the diameter of the normal aorta;
- any saccular protrusion of the aortic wall (as a clear sign of a pathological process).
Risk factors and groups
- Age. Aortic aneurysm most often occurs in people over 60 years of age.
- Tobacco smoking. Smoking is one of the main risk factors for the formation of thoracic aortic aneurysm. With increasing smoking experience, the risk of aneurysm formation increases.
- Arterial hypertension. High blood pressure damages the blood vessels in the body and thereby increases the risk of developing an aortic aneurysm.
- Atherosclerosis. Increased levels of cholesterol and other substances that can damage the inner layer of blood vessels are also an important factor in the formation of aneurysms.
- Floor. Aortic aneurysms occur more often in men than in women. However, women with an aortic aneurysm have a higher risk of rupture than men.
- Race. Aortic aneurysm occurs more often in white people than in people of other races.
- Family history. If someone in the family has had cases of aortic aneurysm, then his blood relatives have an increased risk of developing an aneurysm. These people tend to form aneurysms at a younger age and have a higher risk of rupture.
Clinical picture
The most consistent symptom is abdominal pain. They are usually localized in the umbilical region or in the left half of the abdomen, and can be continuous aching or paroxysmal; sometimes they radiate to the lumbar or groin region; in some patients they are localized mainly in the back. Pain occurs due to the pressure of the aneurysm on the nerve roots of the spinal cord and the nerve plexuses of the retroperitoneal space. Patients often complain of a feeling of increased pulsation in the abdomen, a feeling of heaviness and fullness in the epigastric region, and bloating. Sometimes appetite decreases, nausea, vomiting, belching, constipation, weight loss appear, which is associated with compression of the gastrointestinal tract or with the involvement of the visceral branches of the abdominal aorta in the pathological process. An abdominal aortic aneurysm may be asymptomatic. When examining patients in a horizontal position, increased pulsation of the aneurysm is often detected. Upon palpation in the upper half of the abdomen, most often to the left of the midline, a pulsating tumor-like formation of dense elastic consistency is determined, painless or slightly painful, often motionless. Auscultation over the formation reveals a systolic murmur carried through to the femoral arteries.