All information on the site is for informational purposes only and requires additional consultation with your doctor. Fungal infections affect different parts of the human body. Mycoses are manifested by symptoms:
- inflammation of the skin;
- fragility, thickening, changes in the structure of nails;
- peeling;
- blisters;
- thickening, cracking of the dermis;
- dandruff formation, itching.
Signs of fungus are unpleasant and painful. Anxiety prevents a person from leading a normal lifestyle: studying, working, communicating. After conducting a preliminary examination, doctors choose an individual type of treatment suitable for the patient. The course depends on the severity and location of the pathological process. Manufacturers of pharmaceutical products offer to purchase medicines that have a certain release form: convenient, profitable and effective for the patient. Medicines containing the active ingredient Terbinafine Hydrochloride have proven themselves well among doctors and consumers.
Composition and active substance
Terbinafine contains:
Terbinafine Tablets
1 tablet contains:
Active substance: terbinafine hydrochloride 250 mg
Excipients: microcrystalline cellulose, hyprolose (hydroxypropylcellulose), croscarmellose sodium, colloidal silicon dioxide, calcium stearate, lactose monohydrate.
Terbinafine cream
1 g of cream contains:
Active substance: terbinafine hydrochloride 10 mg
Excipients: benzyl alcohol, polysorbate 60 (Tween 60), sorbitan monostearate, cetyl alcohol, isopropyl myristate, cetyl palmitate, sodium hydroxide, purified water.
Terbinafine ointment
1 g ointment contains:
Active substance: terbinafine hydrochloride 10 mg
Excipients: methyl parahydroxybenzoate (methylparaben), carbomer (rarely cross-linked polyacrylic acid mAPS-06), polysorbate, vaseline oil, propylene glycol, sodium hydroxide (sodium hydroxide), purified water.
Terbinafine spray
Active substance: terbinafine hydrochloride.
Release form
Terbinafine tablets are white or close to white. They have a round, biconvex shape. On one side there is a separation risk. The active substance content in one pill is 250 mg.
Features of the release form depend on the manufacturer. In any case, the tablets are placed in blister packs, which are laid out in cardboard packs.
One cell package can contain 7 tablets, then 1, 2 or 4 such blisters are placed in a pack. Some manufacturers sell 10 pills in cell packaging. Then 1, 2 or 3 blisters are placed in a pack.
pharmachologic effect
Terbinafine is an antifungal agent. Has a wide spectrum of action against fungi that cause diseases of the skin, hair and nails, including:
- dermatophytes such as Trichophyton (e.g. T. rubrum, T. mentagrophytes, T. verrucosum, T. tonsurans, T. violaceum), Microsporum (e.g. M. canis), Epidermophyton floccosum
- yeast fungi of the genus Candida (for example, C. albicans) and Pityrosporum.
In low concentrations, terbinafine has a fungicidal effect against dermatophytes, molds and some dimorphic fungi. Activity against yeast fungi, depending on their type, can be fungicidal or fungistatic.
The mechanism of action is associated with the specific suppression of the early stage of sterol biosynthesis in the fungal cell. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine works by inhibiting the enzyme squalene epoxidase in the cell membrane of the fungus. This enzyme does not belong to the cytochrome P450 system. Terbinafine does not have a significant effect on the metabolism of hormones or other drugs.
Pharmacokinetics
When terbinafine is administered orally, concentrations of the drug are created in the skin, hair and nails, providing a fungicidal effect.
After a single oral dose of terbinafine at a dose of 250 mg, its maximum plasma concentration is reached after 2 hours and is 0.97 mcg/ml. The half-life of absorption is 0.8 hours, and the half-life of distribution is 4.6 hours. Although the bioavailability of terbinafine varies moderately under the influence of food, it is not to such an extent that dose adjustment of the drug is required.
Terbinafine is highly bound to plasma proteins (99%). It quickly penetrates the dermal layer of the skin and concentrates in the lipophilic stratum corneum. Terbinafine also penetrates the secretions of the sebaceous glands, which leads to the creation of high concentrations in the hair follicles, hair and skin rich in sebaceous glands. Terbinafine has also been shown to penetrate the nail plates in the first few weeks after initiation of therapy.
Terbinafine is metabolized quickly and substantially with the participation of at least seven isoenzymes of cytochrome P450, with the main role being played by the isoenzymes CYP2C9, CYP1A2, CYP3A4, CYP2C8 and CYP2C19. As a result of the biotransformation of terbinafine, metabolites are formed that do not have antifungal activity and are excreted primarily in the urine. The terminal half-life is 17 hours. There is no evidence of drug accumulation in the body. There were no changes in the steady-state plasma concentration of terbinafine depending on age, but in patients with impaired renal or hepatic function, the rate of elimination of the drug may be slowed, resulting in higher concentrations of terbinafine in the blood.
When a spray or cream is applied topically, less than 5% of the dose is absorbed, thus the systemic exposure of the drug is minimal.
Fungal skin infections
For mycosis of the feet, smooth skin or scalp, apply the spray once a day. Pre-cleansed infected skin and adjacent healthy areas are thoroughly moisturized (the product is sprayed for 15-30 seconds).
Noticeable improvements in the condition of the skin occur after 3-5 days. If this does not happen, you should consult your doctor; you may need to change the drug. The total duration of continuous therapy is 10-14 days.
Contraindications
- chronic or active liver disease
- chronic renal failure (creatinine clearance less than 50 ml/min)
- children's age (up to 3 years) and with body weight up to 20 kg (for this dosage form)
- lactation period
- lactase deficiency, lactose intolerance, glucose-galactose malabsorption
- hypersensitivity to terbinafine or other components of the drug.
Caution should be used when:
- renal failure (with CC more than 50 ml/min)
- alcoholism
- inhibition of bone marrow hematopoiesis
- tumors
- metabolic diseases
- occlusive diseases of the vessels of the extremities
- cutaneous lupus erythematosus or systemic lupus erythematosus.
Overdose
Cases of overdose are associated, of course, with the use of the tablet form of the drug. Just like side effects, they are extremely rare - only if the patient does not know any boundaries at all and takes the medication in truly unlimited, “horse” doses. During the entire period of use of the drug, only a few cases of overdose were reported, and in each of them the person drank no more than five grams of the drug.
You can think about an overdose if the following symptoms are present: severe headache, dizziness, stomach pain, nausea. Activated carbon (or its analogues in the absence of it), as well as gastric lavage, helps to cope with such a scourge. As for an overdose of any external form of Terbinafine, it is unlikely, moreover, it is practically reduced to zero - except perhaps if Terbinafine in this form is taken orally for some reason.
Terbinafine: instructions for use
Pills
The duration of treatment depends on the indication and severity of the disease.
Children: Orally, after meals, prescribed once a day. A single dose depends on body weight and is: for children weighing less than 20 kg - 62.5 mg (half a 125 mg tablet) from 20 to 40 kg - 125 mg (one 125 mg tablet) over 40 kg - 250 mg (two tablets 125 mg).
Adults: 250 mg once daily in the evening or 125 mg twice daily.
Skin infections:
Recommended duration of treatment:
- tinea pedis (interdigital, plantar or sock-type): 2-6 weeks
- dermatomycosis of the trunk, limbs, legs: 2-4 weeks
- skin candidiasis: 2-4 weeks.
Complete disappearance of the manifestations of infection and complaints associated with it can occur only a few weeks after mycological cure.
Hair and scalp infections:
Recommended duration of treatment:
- mycosis of the scalp: 4 weeks.
Mycoses of the scalp are observed mainly in children.
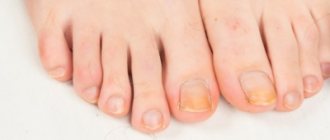
Onychomycosis:
The duration of effective treatment is 6 to 12 weeks in most patients. For onychomycosis of the hands, in most cases, 6 weeks of treatment is sufficient. For onychomycosis of the feet, in most cases, 12 weeks of treatment is sufficient. Some patients who have a reduced rate of nail growth may require longer treatment. The optimal clinical effect is observed several months after mycological cure and cessation of therapy. This is determined by the period of time required for a healthy nail to grow back.
Use of terbinafine in the elderly: There is no evidence to suggest that elderly patients require changes in dosage of the drug or that they experience side effects that differ from those in younger patients. When using the drug in tablet form in this age group, the possibility of concomitant liver or kidney dysfunction should be taken into account.
Cream, ointment
Terbinafine can be applied to the skin 1 or 2 times a day. Before application, it is necessary to clean and dry the affected areas. Apply a thin layer to the affected skin and surrounding areas and rub in lightly. For infections accompanied by diaper rash (under the mammary glands, in the spaces between the fingers, between the buttocks, in the groin area), the places where the cream is applied can be covered with gauze, especially at night.
Average duration of treatment:
- dermatomycosis of the trunk, limbs, legs: 1-2 weeks
- tinea pedis: 2-4 weeks
- skin candidiasis: 1-2 weeks
- tinea versicolor: 2 weeks
- mycosis of the nail plates: 3-6 months.
A decrease in the severity of clinical manifestations is usually observed in the first days of treatment. If treatment is not regular or is stopped prematurely, there is a risk of the infection returning. If after two weeks of treatment there are no signs of improvement, the diagnosis should be verified.
Use of the drug in the elderly: There is no reason to assume that elderly patients require changes in the dosage of the drug or that they experience side effects that differ from those in younger patients.
Children: Experience in children is limited.
Spray
Orally for adults - 250 mg/day in 1 or 2 doses. The duration of treatment depends on the indications and severity of the infection: for skin lesions - 2-4 weeks, for nail lesions - from 6 weeks to 4 months or more. Children weighing more than 40 kg - 250 mg/day, 20-40 kg - 125 mg/day, up to 20 kg - 62.5 mg/day.
Apply externally 1-2 times a day for 1-2 weeks.
Indications for use
- all forms of release: treatment and prevention of fungal skin lesions caused by infection with Trychophyton spp. (T. rubrum, T. mentagrophytes, T. verrucosum, T. tonsurans, T. violacium), Epidermophyton floccosum, Microsporum spp. (M. canis, M. gypseum): inguinal athlete's foot, mycoses of the feet and smooth skin of the body, etc.;
- tablets: mycoses of the nails (onychomycosis) and the scalp (microsporia, trichophytosis), as well as severe widespread dermatomycosis of the smooth skin of the trunk and extremities, requiring systemic treatment;
- tablets and cream: yeast skin infections caused by Candida fungi; in particular, skin diaper rash (cream) and candidiasis of the mucous membranes (tablets);
- cream and spray (topicals): pityriasis versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (also known as Malassezia furfur).
special instructions
Irregular use of terbinafine or premature cessation of treatment may lead to relapse of the disease. The duration of therapy can be influenced by factors such as the presence of concomitant diseases, the condition of the nails with onychomycosis at the beginning of the course of treatment. If after 2 weeks of treatment of a skin infection there is no improvement in the condition, it is necessary to re-determine the causative agent of the disease and its sensitivity to the drug. Systemic use for onychomycosis is justified only in the case of total damage to most nails, the presence of severe subungual hyperkeratosis, and the ineffectiveness of previous local therapy. When treating onychomycosis, a laboratory-confirmed clinical response is usually observed several months after mycological cure and cessation of treatment, which is due to the rate of regrowth of a healthy nail. Removal of nail plates is not required when treating onychomycosis of the hands for 3 weeks and onychomycosis of the feet for 6 weeks. In the presence of liver disease, the clearance of terbinafine may be reduced. During treatment, it is necessary to monitor the activity of liver transaminases in the blood serum. In rare cases, cholestasis and hepatitis occur after 3 months of treatment. If signs of liver dysfunction occur (weakness, persistent nausea, loss of appetite, excessive abdominal pain, jaundice, dark urine or discolored stools), the drug should be discontinued. Prescribing terbinafine to patients with psoriasis requires caution, because in very rare cases, terbinafine can cause an exacerbation of psoriasis. When treating with terbinafine, general hygiene rules should be observed to prevent the possibility of re-infection through underwear and shoes. During treatment (after 2 weeks) and at the end it is necessary to carry out antifungal treatment of shoes, socks and stockings.
Avoid contact with eyes as it may cause irritation. If the drug accidentally gets into your eyes, they should be immediately rinsed with running water, and if persistent irritation develops, you should consult a doctor. If allergic reactions develop, the drug must be discontinued.
Side effects
Using Terbinafine may cause side effects. These include a possible allergic reaction. It can be expressed by a rash or hives. In rare cases, treatment leads to angioedema, anaphylactoid reactions, Lyell's or Stevens-Johnson syndrome.
The digestive system often reacts to Terbinafen therapy with a feeling of a full stomach, decreased appetite, dyspeptic disorders, and abdominal pain. Less commonly, treatment leads to temporary loss of taste, jaundice, cholestasis, and hepatitis.
The musculoskeletal system may respond to treatment with myalgia or arthralgia. Adverse reactions of the nervous system usually result in headaches. In some cases, therapy provokes paresthesia, hyposthesia, dizziness, depression, and anxiety.
Compatibility with other drugs
Interactions that interfere with the metabolism of other drugs
Inhibits the CYP2P6 isoenzyme and interferes with the metabolism of drugs such as tricyclic antidepressants and selective serotonin reuptake inhibitors (for example, desipramine, fluvoxamine), beta blockers (metoprolol, propranolol), antiarrhythmics (flecainide, propafenone), monoamine oxidase B type inhibitors (for example, selegiline) and antipsychotic (eg, chlorpromazine, haloperidol) agents.
Interactions that accelerate the elimination of terbinafine
Drugs that are inducers of cytochrome P 450 isoenzymes (for example, rifampicin) can accelerate the elimination of terbinafine from the body.
Interactions that slow down the metabolism and elimination of terbinafine
Medicines that are inhibitors of cytochrome P 450 isoenzymes (for example, cimetidine) can slow down the metabolism and excretion of terbinafine from the body. If these drugs are used concomitantly, a dose adjustment of terbinafine may be required.
Menstrual irregularities are possible when taking terbinafine and oral contraceptives simultaneously.
Terbinafine reduces the clearance of caffeine by 21% and prolongs its half-life by 31%. Does not affect the clearance of phenazone, digoxin, warfarin.
When used together with ethanol or drugs that have hepatotoxic effects, there is a risk of developing drug-induced liver damage.
Use during pregnancy and lactation
During pregnancy, the drug can be used if the required benefit from treatment is greater than the harm caused.
When breastfeeding, the appearance of concentrations in breast milk should be taken into account.
Therefore, it is advisable to transfer the baby to artificial feeding during the treatment period.
Such measures can be explained by the fact that the effect of the antifungal agent on the baby’s body has not been fully studied.
It is also necessary to strictly avoid contact of Terbinafine cream with the baby’s skin. It is also contraindicated to treat nipples with an antifungal agent when breastfeeding to avoid the medicine getting into the baby's stomach.
Treatment with an antifungal agent during pregnancy and lactation is no different from the above rules for antifungal treatment.
Analogues and prices
Among foreign and Russian analogues Terbinafine are:
Exifin. Manufacturer: Dr. Reddy's (India). Price in pharmacies from 854 rubles. Lamisil uno. Manufacturer: Novartis (Switzerland). Price in pharmacies from 937 rubles. Lamisil dermgel. Manufacturer: Novartis (Switzerland). Price in pharmacies from 571 rub. Thermikon. Manufacturer: Pharmstandard (Russia). Price in pharmacies from 358 rubles. Lamisil. Manufacturer: Novartis (Switzerland). Price in pharmacies from 2068 rubles.
"Terbinafine": reviews
It is impossible to avoid both negative and positive opinions about any medicine, since the medicine helps some, but not others (for various reasons). From the favorable reviews of Terbinafine, you can find out that the drug acts quite quickly and, most importantly, effectively. People write that they struggled with the fungus for quite a long time, but nothing could save them - until the very moment they tried Terbinafine. Reviews of the drug, by the way, also contain comments that in some mild cases, at the initial stage of the disease, external dosage forms cope well with it; As for the tablets, they have a good therapeutic effect in severe advanced situations.
If we talk about positive opinions, then, of course, those who used the medicine are pleased with its price. In addition, among the positive reviews about Terbinafine are words about the drug’s immediate elimination of unpleasant odor from the feet.
There is no such thing as a fly in the ointment. Thus, people note that the drug did not help against nail fungus. In addition, in negative reviews about Terbinafine, people notice that the medication has only a temporary effect and does not completely eliminate the problem.