Diagnostics
2.1 Complaints and anamnesis
A variety of onset options is characteristic, polyarthritis predominates, less often joint damage is preceded by the predominance of arthralgia, morning stiffness, deterioration of general condition, weakness, weight loss, low-grade fever, lymphadenopathy.
Opening options:
- Symmetrical polyarthritis with months of increasing pain and stiffness, in 50% of small joints of the hands.
- Acute polyarthritis with predominant damage to the joints of the hands and feet, severe morning stiffness, often with RF in the blood.
- Mono-oligoarthritis of the knees or shoulders with rapid involvement of the small joints of the hands and feet.
- Acute monoarthritis of large joints.
- Acute oligo- or polyarthritis with systemic phenomena, more often in young people.
- “Palindromic rheumatism”, which ends in complete recovery after a few hours or days.
- Recurrent bursitis and tenosynovitis, predominantly of the wrist.
- Acute polyarthritis in the elderly - “RS3PE syndrome”.
- Generalized myalgia.
- Undifferentiated arthritis (UDA) with uncharacteristic clinical manifestations.
Frequent clinical variants of NDA:
- Oligoarthritis of large joints;
- Asymmetric arthritis of the joints of the hands;
- RF seronegative oligoarthritis of the joints of the hands;
- Unstable polyarthritis.
2.2 Physical examination
The diagnosis of RA is made based on characteristic clinical signs.
Peripheral arthritis is manifested by pain, swelling, and limited mobility.
Typically, manifestations in the carpal PJF and PIP may prevail over damage to the PJF or large joints.
Joint damage
Characteristic manifestations in the debut:
- Pain and swelling of the affected joints;
- Weakening the grip force of the hand;
- Morning stiffness;
- Rheumatoid nodules (rare).
Characteristic manifestations in the advanced and final stages:
- Hands: ulnar deviation; “boutonniere” or “swan neck” fingers; “lornette” type deformation.
- Knees: flexion and valgus deformity, Baker's cyst.
- Feet: subluxation of the heads, lateral deviation, deformity of the big toe.
- Cervical: atlantoaxial subluxation, rarely with compression of the spinal cord or vertebral artery.
- Cricoid-arytenoid joint: deepening of voice, shortness of breath, dysphagia, recurrent bronchitis.
- Ligamentous apparatus and synovial bursae: wrist tenosynovitis; elbow; Baker's cyst.
- Extra-articular manifestations.
- Constitutional: generalized weakness, malaise, weight loss, low-grade fever.
- CVS: pericarditis, vasculitis, valvular granulomatosis (very rare), early atherosclerosis.
- Lungs: pleurisy, interstitial disease, bronchiolitis obliterans, Kaplan syndrome.
- Skin: rheumatoid nodules, thickening and wasting; digital arteritis, microinfarctions of the nail bed, livedo reticularis.
- Nervous system: compression, symmetrical sensory-motor neuropathy, multiple mononeuritis (vasculitis), cervical myelitis.
- Muscles: generalized amyotrophy.
- Eyes: keratoconjunctivitis sicca, peripheral ulcerative keratopathy, episcleritis, scleritis, scleromalacia.
- Kidneys: amyloidosis, vasculitis, nephritis (rare).
- Blood: anemia, thrombocytosis, neutropenia.
Special clinical forms
Felty's syndrome:
- neutropenia;
- splenomegaly;
- hepatomegaly;
- severe joint damage;
- extra-articular manifestations (vasculitis, neuropathy, pulmonary fibrosis, Sjogren's syndrome);
- hyperpigmentation of the skin of the lower extremities;
- high risk of infectious complications.
Adult Still's disease:
- recurrent febrile fever;
- arthritis with high laboratory activity, seronegative for the Russian Federation;
- maculopapular rash.
2.3 Laboratory diagnostics
- General blood analysis
- BAC: AST and ALT, creatinine, glucose
- Markers of hepatitis B, C, HIV viruses
- Pregnancy test
- Lipid profile
- Antinuclear factor (ANF)
- IgG concentration
The main diagnostic biomarkers of RA are rheumatoid factor (RF) and antibodies to citrullinated proteins (ACP).
A sensitive, but insufficiently specific marker of RA is IgM RF , high titer - with rapidly progressive destruction of joints and systemic manifestations.
ACCP is a more specific and informative marker of RA, especially at an early stage.
Determination of ESR - a highly sensitive, but nonspecific and unstable marker of systemic inflammation.
Determination of CRP is used to assess the activity of inflammation, predict the rate of joint destruction and differentiate between RA and SLE. CRP is more stable than ESR.
Increases in ESR and CRP are included in the classification criteria for RA (ACR/EULAR, 2010) and components of RA activity indices.
Potential risk factors for adverse reactions (or complications) of pharmacotherapy:
- markers of chronic infections;
- cardiovascular diseases;
- renal dysfunction;
- drinking alcohol;
- turn of the Mantoux tuberculin test.
2.4 Instrumental diagnostics
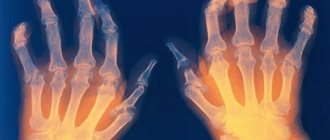
Plain radiography of the hands and feet - during the initial examination and then annually, at stages 3 and 4, as necessary.
Characterized by multiple and symmetrical lesions of the small joints of the hands and feet.
In case of acute onset and active inflammation:
- after 1 month of illness - periarticular osteoporosis and single cysts;
- after 3 to 6 months - multiple cysts, narrowing of the cracks and single erosions.
Early radiological signs of arthritis are detected in the joints:
- 2 and 3 metacarpophalangeal;
- 3 proximal interphalangeal;
- wrists;
- wrist;
- styloid processes of the ulna;
- 5 metatarsophalangeal.
Typical for RA are symmetrical radiographic changes in the joints:
- metacarpophalangeal,
- proximal interphalangeal;
- wrists;
- metatarsophalangeal;
- 1st interphalangeal feet.
For stages 3 and 4 of change:
- distal interphalangeal joints of the hands,
- proximal interphalangeal joints of the feet.
RA never begins with a lesion:
- distal interphalangeal joints of the hands and feet;
- proximal interphalangeal joints of the feet.
Bone ankylosis exclusively:
- in the intercarpal joints;
- 2-5 carpometacarpal joints;
- rarely in the tarsal joints.
Bone ankylosis never occurs in RA:
- interphalangeal;
- metacarpophalangeal;
- metatarsophalangeal;
- DOS;
- 1st carpometacarpal.
X-ray changes characteristic of RA:
- large joints of the upper and lower extremities;
- absent in the joints of the axial skeleton.
Features of radiological changes in joints in RA in old age
- Periarticular osteoporosis can be postmenopausal.
- Osteoarthritis is possible in the DIP and PIP joints of the hands, large joints, and less commonly in the PJF. With RA, erosions in typical joints of the wrist, PLF, PJF.
- X-ray of large joints in RA - only if osteonecrosis, septic arthritis, etc. is suspected.
Chest X-ray to identify rheumatoid respiratory lesions during the initial examination and then annually.
CT scan of the lungs to identify diffuse or focal lesions and differential diagnosis.
MRI of joints is a more sensitive method for detecting arthritis at the onset of RA than standard radiography of joints:
- MRI signs of arthritis are nonspecific, but they help predict the progression of joint destruction.
- MRI of the hands is indicated when it is difficult to diagnose early RA and LDA and to assess the prognosis.
Ultrasound of the joints allows one to judge the severity of inflammation and predict the duration of remission.
- MRI and ultrasound of joints are not recommended to confirm the diagnosis or select therapeutic tactics.
X-ray assessment of the progression of joint destruction:
- every 6-12 months for the first 3 years in early RA,
- Once a year for advanced RA.
2.5.Other diagnostics
Functional impairment is assessed over time using the Health Assessment Questionnaire (HAQ)
Consultations:
- cardiologist – in case of complaints from the cardiovascular system, to prescribe/correct cardioprotective therapy;
- endocrinologist – diagnosis of diseases affecting the course of RA;
- gastroenterologist/endoscopist – when prescribing NSAIDs;
- phthisiatrician – when prescribing a biologically active drug and suspected latent tuberculosis.
Features of manifestation
The disease manifests itself in different ways. The clinical picture of rheumatoid arthritis depends on:
- stages of the disease;
- localization of inflammatory and destructive processes;
- severity of joint damage;
- presence of complications.
With a latent course of the disease, the patient has complaints about:
- chronic fatigue;
- muscle weakness;
- rapid weight loss;
- excessive sweating;
- unreasonable increase in body temperature (mainly in the morning);
Subacute rheumatoid arthritis is characterized by the appearance of pain. The patient is bothered by aching pain in the area of inflamed joints. Their intensity is highest in the evening. It is possible to reduce the severity of pain only by using NSAIDs.
Different types of joints are involved in inflammatory processes. But those most often affected are those responsible for the mobility of the knees, fingers and wrists. Sometimes inflammation of the tissues of the shoulders, hips, and spinal column is observed.
| Joint inflammation due to arthritis | Characteristic symptoms |
| Carpal, interphalangeal | Swelling of the tendons located near the affected joints |
| Impaired hand mobility | |
| Difficulty making a fist with your fingers | |
| Decreased sensitivity in the first three fingers | |
| Elbow, radioulnar | Elbow pain |
| Deterioration of joint mobility (especially after a long stay in one position) | |
| Shoulder | Reduction of mass, muscle dysfunction in the neck, shoulders and collarbone |
| Increased body temperature at the lesion site | |
| Tissue swelling | |
| Limitation of joint mobility | |
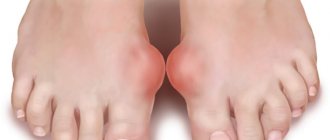
| Ankle | Toe misalignment |
| Pain in legs when walking or running | |
| Change in gait | |
| Knees | Quadriceps dysfunction |
| Decreased mobility in the knee | |
| Bulging of intra-articular fluid into the popliteal fossa | |
| Hip | Pain radiating to the groin |
| Intermittent claudication | |
| Necrosis of the femur | |
| Joints of the cervical spine | Discomfort, pain in the neck, arm and shoulder area |
| Headache | |
| Crunching, displacement of cervical vertebrae | |
| Neck muscle stiffness |
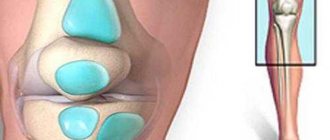
This diagram shows two joints - healthy and damaged. Read it carefully.
In rheumatoid arthritis, the joints are first affected. But if the disease progresses, the functioning of the following body systems is disrupted:
Digestive . Associated symptoms of arthritis:
- loss of appetite;
- flatulence;
- pain in the stomach, lower abdomen.
Cardiovascular:
- inflammation of the pericardial sac;
- granulomatous lesions of the heart valves (rarely observed);
- atherosclerosis.
Urinary . Signs of progressive rheumatoid arthritis:
- glomerulonephritis;
- amyloidosis;
- renal failure.
Nervous . Rheumatoid arthritis is characterized by:
- decreased sensitivity at the site of the lesion;
- the occurrence of cervical myelitis, paralysis;
- disruption of heat exchange processes.
Hematopoietic:
- anemia;
- deviation of blood counts from the norm (decrease in the number of platelets, leukocytes).
Respiratory . Manifestations of systemic autoimmune disease:
- damage to the lungs by rheumatoid nodules (Kaplan syndrome);
- bronchiolitis.
Visual:
Treatment
The goal is stable clinical remission or, at a minimum, persistently low RA activity.
Principles of “Treat to target”:
- anti-inflammatory therapy from the moment of diagnosis;
- frequent - every 3 months. until remission, every 6 months. in remission, objective monitoring of the condition;
- changing the treatment regimen in the absence of a sufficient response to therapy, followed by constant dynamic monitoring.
RA treatment is carried out throughout the patient's life.
3.1 Conservative treatment
The basis of RA treatment is drug therapy:
- NSAIDs,
- glucocorticoids (GC),
- basic anti-inflammatory drugs (DMARDs).
The effectiveness of therapy is assessed every 1–3 months until remission, then every 3–6 months according to ACR/EALAR criteria (2011).
A decrease in RA activity in the first 3 months of treatment is associated with the development of remission after 12-24 months.
Treatment is carried out by a rheumatologist, or, as an exception, by a general practitioner, but with the advisory support of a rheumatologist.
A multidisciplinary approach reduces the negative impact of comorbid pathology on the course, increases the effectiveness of pharmacotherapy and improves the prognosis.
Patients should avoid factors that provoke exacerbation: intercurrent infections, stress, smoking, obesity and periodontitis.
Regular physical activity improves functional status and helps maintain ability to work.
(NSAID)
NSAIDs have a satisfactory symptomatic (analgesic) effect without affecting the activity of inflammation, the progression of joint destruction and cause severe adverse reactions (AR).
The choice of NSAIDs is largely determined by their safety rather than their effectiveness.
DMARD therapy is indicated for all patients and is prescribed as early as possible, no later than 3–6 months. from the moment symptoms of joint damage appear.
(Methotrexate)
The first-line drug methotrexate (MTX for all patients , except those planning pregnancy.
The prescription of MT is individualized, starting at 10–15 mg/week and increasing every 2–4 weeks. by 2.5–5 mg to 25–30 mg/week. under the control of ALT/AST, creatinine, UAC every 1–1.5 months until a stable dose of MT, then every 3 months.
Increased ALT/AST:
- 3 times higher than the upper limit of normal (ULN) - interrupt MT until normalization, then MT at a lower dose;
- with a persistent increase in the level >3 ULN, adjust the dose of MT;
- If an increase of >3 ULN persists after discontinuation of MT, carry out diagnostics.
24 hours after taking MTX, at least 5 mg/week is recommended. folic acid .
If MTX tablets are insufficiently effective or poorly tolerated, the patient is transferred to subcutaneous MTX injections until changing DMARDs and prescribing a GEBD.
For “active” RA with DAS28 more than 5.1 and MT more than 15 mg, subcutaneous administration is recommended.
Risk factors for ADR on MT:
- refusal to take folic acid,
- concomitant diabetes mellitus,
- obesity,
- hyperlipidemia,
- intercurrent infections,
- elderly age,
- excessive alcohol consumption.
(Combined therapy with DMARDs)
In active RA with an unfavorable prognosis and resistance to 3-month MTX monotherapy at the maximum effective dose, a combination of MTX with standard DMARDs with or without GC is prescribed.
If MTX is contraindicated or poorly tolerated, 20 mg/day leflunomide (LEF) or 3-4 g/day sulfasalazine (SULF) is prescribed.
Hydroxychloroquine (HCQ) is recommended only in combination with MTX.
SULF and HCQ can be used during pregnancy, with LEF blood pressure control is necessary, with all DMARDs it is necessary to control CBC, AST, ALT and creatinine
Glucocorticoids (GC)
Prescribed only by a rheumatologist.
Duration of taking GC in combination with MT or other DMARDs:
- in early RA before the development of the effect of DMARDs (bridge therapy);
- for exacerbation at any stage of RA with a short course.
GK is canceled as quickly as possible, preferably no later than 6 months from the start.
Monotherapy with GC is not recommended.
Specific to GC is the development of HP: osteoporosis, insulin resistance, infectious complications and cardiovascular accidents; but may be a consequence of uncontrolled “rheumatoid” inflammation.
At a dose higher than 5 mg/day of GC for longer than 3 months, prophylactic administration of calcium and vitamin D supplements and antiosteoporetic therapy are necessary.
(Genetically engineered biological products - GIBP)
Prescribed in case of poor tolerability or insufficient effectiveness of 3-month therapy with MTX or MTX + DMARDs.
After 3 months of DMARDs, a “moderate” effect with a decrease from the initial DAS28 of more than 1.2 points is grounds for continuing for another 3 months.
Targeted synthetic drugs (ts-DMARDs or TOFA) are prescribed in a similar way to GEBPs.
To increase the effectiveness of GIBP, a combination with MT is advisable .
Equally effective:
- MT or MT + DMARDs followed by the prescription of a GEBD if the result is insufficient (step-up);
- MT+ GIBP (“induction”) from debut;
In case of contraindications and poor tolerability of MT and DMARDs, monotherapy with GEBD or TOFA is possible.
The drug of choice for monotherapy of GEBD is tocilizumab ( TCZ).
If the “first” GIBP is insufficiently effective after 6 months, “switch” to another GIBP with a different mechanism of action or TOFA:
- abatacept (ABC),
- adalimumab (ADA),
- golimumab (GLM),
- infliximab (INF),
- rituximab (RTM),
- etanercept (ETC).
If the “second” GEBD is insufficiently effective, TOFA or MTX are advisable in the “third line” if adequate MTX therapy has not previously been carried out.
Contraindications to GIBP:
- Active and severe infections;
- Hypersensitivity to GIBP;
- Immunodeficiency conditions;
- Liver failure: ALT and AST > 5 x ULN;
- Severe hematological disorders: leukopenia, thrombopenia, neutropenia;
- Multiple sclerosis;
- SLE;
- Heart failure III-IV NYHA;
- History of cancer.
Adverse reactions to GIBP
- Immediate NR: standard infusion (SIR); anaphylaxis; associated with the synthesis of anti-drug antibodies (ALA);
- Infections;
- Related to immune mechanisms: hematological; cardiovascular; pulmonary non-infectious; gastrointestinal; metabolic; skin; neurological.
For latent tuberculosis, 2 months of preventive treatment with isoniazid or rifampicin is indicated before starting a biological therapy; the risk of reactivation against the background of biological therapy is:
- high: INF, ADA, GLM and CZP;
- moderate: ETC, ABC, TCZ;
- low: RTM
Risk factors for infectious complications:
- Comorbid diseases (kidneys and lungs);
- Elderly age;
- GK therapy
INF is more likely to cause severe infections than ETC and ADA The risk of infectious complications with ABC is lower than with TNF-a inhibitors.
All GEBDs cause psoriasis, increased liver enzymes ( TCZ is rare), and cytopenias.
Against the background of RTM, the risk of liver failure associated with the hepatitis B virus and progressive multifocal leukoencephalopathy (regimen with immunosuppressants) is increased.
During treatment with TCZ there is a risk of intestinal perforation.
There is no evidence of an increase in the incidence of lymphomas and skin cancer in the presence of biological therapy.
Preliminary recommendations for choosing GIBP
The choice of route of administration of GEBP (IV or SC) depends on patient preference.
Monoclonal antibodies to TNF-a (ADA, INF, GLM and CZP) are preferred in RF/ACB seronegative patients with concomitant diseases.
ETC:
- induces uveitis;
- preferable for those at risk of tuberculosis activation, those planning pregnancy, and asymptomatic carriers of the hepatitis C virus.
RTM:
- as a “first” biologic drug, it is highly effective in RF/ABC-positive RA and cryoglobulinemic vasculitis associated with carriage of the hepatitis C virus;
- preferable in patients with lupus-like syndrome, Felty or Schoengren's syndrome, and stage III-IV heart failure;
- contraindicated in autoimmune disorders, cancer with a history of less than 10 years, risk of reactivation of latent tuberculosis, demyelinating diseases of the central nervous system;
- to maintain the effect after 6 months, a repeated course of RTM (possibly 500 mg x 2);
- during therapy, serum IgG monitoring;
- high risk of SIR requires premedication 60 minutes before infusion of 100 mg 6-MPRED IV + 1 g paracetamol + 10 mg chlorphenamine IV.
A B C:
- as a “first” GIBP, it is highly effective in the RF/ABC positive variant of RA;
- lower risk of infectious complications, reactivation of latent tuberculosis infection, exacerbation of ILD and cardiovascular complications;
- preferred in patients with lupus-like syndrome, Felty or Schoengren's syndrome, and stage III-IV heart failure.
TCZ:
- as the “first” GEBD in patients with clear constitutional manifestations of RA and laboratory abnormalities;
- monotherapy is highly effective in case of contraindications or poor tolerance to MT;
- effective for adult Still's disease;
- response to 3 months of primary therapy can predict effectiveness;
- relatively safe during pregnancy;
- requires monthly monitoring of neutrophils, platelets, liver enzymes, lipid profile in the first 6 months of therapy;
- treatment is stopped when neutrophils 9/L, platelets 3/L and liver enzymes >5 ULN;
- Careful monitoring of infectious complications is necessary.
Treatment tactics after achieving remission
Gradual reduction (“titration”) of the dose or discontinuation of GIBP when remission is achieved and GC is discontinued or taken
To maintain remission after dose reduction (or discontinuation) of GIBD, adequate therapy with MTX is carried out.
In the event of an exacerbation against the background of a dose reduction (or discontinuation) of GEBD, re-administration of the same or other GEBD in a standard dose suppresses the activity of inflammation in the majority.
Cancellation of a biologic drug in cases of advanced RF/ACCP positive variant of RA often leads to exacerbation.
Reducing the dose or discontinuing standard DMARDs - with long-term stable remission for more than 12 months after discontinuation of the steroid.
Discontinuation of standard DMARDs in patients with advanced RA often leads to exacerbation of the disease.
3.2. Surgery
Surgical treatment is carried out in specialized traumatology and orthopedic departments.
Indications for surgical treatment:
- resistant synovitis;
- joint deformity;
- persistent pain syndrome;
- dysfunction of the joint.
Surgical interventions:
- arthroscopic and open synovectomy;
- debridement;
- osteotomy;
- osteoplasty;
- joint replacement.
MTX treatment is relatively safe and is carried out in the perioperative period; discontinuation of MTX can cause exacerbation of RA.
In case of severe renal impairment, a break in the use of MT is advisable.
The use of GIBP is suspended for a while and resumed when the surgical wound has healed satisfactorily.
Treatment with GC should be continued at the same dose.
On the day of surgery, replacement therapy with 25-100 mg hydrocortisone IV infusion or 5-30 mg 6-MPRED is required.
3.3. Other treatment
Educational programs help maintain work capacity and functional status, help manage pain, and reduce disability.
Training is carried out at different periods of the disease and is based on the cognitive-behavioral model.
Individually oriented educational programs are recommended.
Who treats rheumatoid arthritis?
Diagnosing and treating rheumatoid arthritis requires a team effort involving you and several healthcare professionals who specialize in different areas.
- Rheumatologists who specialize in arthritis and other diseases of the bones, joints and muscles.
- Primary care physicians, such as internists, who specialize in diagnosing and treating adults.
- Orthopedists who specialize in the treatment and surgery of diseases or injuries of bones and joints.
- Physiotherapists who help improve joint function.
- Occupational therapists who teach ways to protect joints minimize pain.
- Dietitians who teach nutrition to improve health and maintain a healthy weight.
- Mental health professionals who help people cope with psychological difficulties.
Rehabilitation
Exercise therapy is recommended.
Occupational therapy (ET) is recommended as an adjunct to drug treatment.
Long-term functional status is improved by training in joint protection methods, energy saving strategies, changing behavioral patterns, and the use of assistive devices.
Orthosis of the hand and wrist joint has a positive effect on pain and hand function.
Foot care and orthotics are effective.
Data on the effectiveness of physical therapy for RA are contradictory.
Balneotherapy (BT) is an additional method of treating RA, used for polyarthritis with low activity.
Causes of rheumatoid arthritis
Researchers don't know what causes the immune system to go against the body's joints and other tissues. Research shows that a combination of the following factors can lead to the disease:
- Genes . Certain genes that affect the immune system can lead to rheumatoid arthritis. However, some people who have these genes never develop the disease. This suggests that genes are not the only factor in the development of RA. In addition, the presence of more than one gene can determine who gets the disease and how severe it becomes.
- Environment. Researchers continue to study how environmental factors may trigger rheumatoid arthritis in people who have certain genes that also increase their risk. Additionally, certain factors, such as bacteria, viruses, and gum disease, may play a role in the development of RA.
- Sex hormones . Researchers believe that sex hormones may play a role in the development of rheumatoid arthritis when genetic and environmental factors are also involved.
Research shows:
- Women are more likely than men to develop rheumatoid arthritis.
- The disease may improve during pregnancy and worsen after pregnancy.
Additional information affecting the course and outcome of the disease
Comorbidity
High RA activity is associated with an increased risk of cardiovascular pathology, and effective therapy reduces the risk of cardiovascular pathology.
Long-term use of GCs is associated with an increased risk of cardiovascular complications and mortality.
In case of FC III-IV CHF (NYHA), due to the risk of decompensation, TNF-a inhibitors are not recommended.
Against the background of antiviral therapy for hepatitis B, DMARDs and GEBD are possible (preferably ETC and ABC).
For carriers of the hepatitis C virus who are not receiving antiviral therapy, SULF and HCQ are recommended, but not MTX and LEF; the results of the use of biologically active drugs are contradictory.
When treating cancer, DMARDs (with the exception of HCQ and SULF) and GEBD are interrupted.
If there is a history of non-melanoma skin cancer and solid tumor, DMARDs are recommended; GEBDs are used with caution.
If you have a history of lymphoproliferative diseases, you can take HCQ, SULF, RTM; treatment with TNF-a inhibitors is not recommended; other DMARDs and GEBD are prescribed with caution.
Vaccination
DMARDs and biologically active drugs suppress post-vaccination immunity
Before starting treatment, vaccination is recommended:
- From (inactivated) influenza and pneumococcal infections to all patients;
- For hepatitis B in high-risk groups;
- For herpes zoster in patients over 60 years of age.
Vaccination with live vaccines is contraindicated against the background of a biologically transmitted disease:
- measles,
- rubella,
- mumps,
- polio,
- rotaviruses,
- yellow fever, etc.
After discontinuation of the GIBD, vaccination is carried out:
- after 1 month ETC;
- after 3 months ABC, ADA, TCZ;
- after 6 months INF;
- after 12 months RTM.
Establishing diagnosis
It is advisable to make a diagnosis of RA in the first 1-3 months from the onset of the first symptoms of the disease.
When making a diagnosis of RA, you must:
- identify one swollen joint;
- exclude other diseases with inflammation of the joints;
- identify 6 signs out of 10 possible in 4 positions, joint damage characteristic of RA and laboratory abnormalities.
Examples of formulation of clinical diagnoses:
- Rheumatoid arthritis seropositive (M05.8), advanced stage, activity II, erosive (radiological stage II), with systemic manifestations (rheumatoid nodules), ACCP (-), FC II.
- Rheumatoid arthritis seronegative (M06.0), early stage, activity III, non-erosive (X-ray stage I), ACCP (+), FC I.
- Rheumatoid arthritis seropositive (M05.8), late stage, erosive (radiological stage III), activity II, with systemic manifestations (rheumatoid nodules, digital arteritis), ACCP (? - not studied), FC III, complications - right carpal tunnel syndrome , secondary amyloidosis with kidney damage.
- Probable rheumatoid arthritis (M06.9), seronegative, early stage, activity II, non-erosive (radiological stage I), ACCP (+), FC I.
The role of the disease in modern society
Rheumatoid arthritis is a severe pathological condition that occurs with periods of exacerbation and remission. The acute phase, according to clinical recommendations, is always accompanied by severe pain and inflammation. These symptoms significantly impair the performance and general condition of patients. Periods of subsiding exacerbation are characterized by the absence or mild severity of symptoms of inflammation. The prevalence of rheumatoid arthritis, according to the latest clinical recommendations, among the general population is about 1-2%. The disease most often begins in middle age (after 40 years), but all age groups can be affected (for example, juvenile rheumatoid arthritis). Women get sick 1.5-2 times more often than men.
By contacting a specialist at the initial stage of the disease, proper diagnosis and timely treatment, as well as following all the doctor’s recommendations, you can maintain remission of the disease for several years and delay the loss of ability to work and physical activity for many years.
The timing of treatment starts plays a very important role in predicting rheumatoid arthritis. The sooner the diagnosis occurs and medications are taken, the easier the disease progresses, and long periods of remission occur more often. With late diagnosis of the disease, there is a high probability of early disability and rapid destruction of joints.
Pathogenesis
Rheumatoid arthritis is classified by specialists as an autoimmune disease. This group of diseases is characterized by the behavior of protective cells - lymphocytes. They, instead of actively diagnosing foreign bacteria, fungi, viruses, and destroying them, begin to attack their own healthy cells[11]. This pathological process of disruption of the interaction of cells of the immune system in the immune response consists of the following stages:
- Synoviocytes acquire the features of macrophages, secrete proinflammatory cytokines, primarily tumor necrosis factor alpha, interleukin 1, become antigen-presenting cells and cause activation of T-helper type 1 cells.
- In the cells of the synovial fluid and in the synovium of the joint, a large number of T-helper type 1 cells appear, secreting interferon gamma and activating macrophages.
- Activated macrophages and monocytes produce pro-inflammatory cytokines: tumor necrosis factor alpha, IL-1, IL-6.
- An increase in the concentration of IL-8 in the synovial fluid causes a high concentration of neutrophils in it.
- IL-1 causes fever, activation of osteoclasts, which contributes to osteoporosis of the subchondral plate of bone. Tumor necrosis factor causes the appearance of adhesion molecules on the surface of endothelial cells, promoting exudation, causing weight loss, anemia of chronic inflammation. I16, activating hepatocytes, causes an increase in their production of C-reactive protein; activates B lymphocytes (transforming them into plasma cells).
- In the blood, the concentration of plasma cells producing immunoglobulins increases significantly.
- In the blood and synovial fluid of 80% of patients, the concentration of IgM and IgG to the altered Fc region of IgG sharply increases (rheumatoid factors).
- The release of endothelial growth factor promotes the proliferation of capillaries in synovial tissue. Angioneogenesis and proliferation of active fibroblasts and synoviocytes lead to the formation of pannus - aggressive tissue that has signs of tumor-like growth, capable of penetrating into cartilage, the articular surface of the bone, forming erosions, and into the ligamentous apparatus. It is important to note that the clone of uncontrollably multiplying, aggressive synoviocytes that makes up the pannus is formed relatively late - several months after the onset of the disease.
- The formation of immune complexes in the blood as a result of the interaction of IgG with rheumatoid factors leads to complement activation and damage to the microvasculature, which explains the visceral manifestations of rheumatoid arthritis.
In the later stages of rheumatoid arthritis, proliferative processes (growth of pannus) may not depend on autoimmune mechanisms and are maintained autonomously.[12]