| Levofloxacin | |
| Levofloxacin | |
| Levofloxacin | |
| Chemical compound | |
| IUPAC | (-)-(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de] 1,4-Benzoxazine-6-carboxylic acid hemihydrate |
| Gross formula | C₁₈H₂₀FN₃O₄ |
| Molar mass | 361.368 g/mol |
| CAS | 100986-85-4 |
| PubChem | 149096 |
| DrugBank | APRD00477 |
| Classification | |
| ATX | J01MA12 S01AX19 |
| Pharmacokinetics | |
| Bioavailable | 99% |
| Plasma protein binding | 24 to 38% |
| Metabolism | Kidneys |
| Half-life | from 6 to 8 hours |
| Excretion | With urine |
| Dosage forms | |
| solution for infusion, film-coated tablets, film-coated tablets, eye drops | |
| Method of administration | |
| Oral, ophthalmic | |
| Other names | |
| Glevo, Levaquin, Levolet, Levofloxacin, Leflobact, Levoflocin FS, Lefokcin, L-Optik Rompharm, Maklevo, Oftaquix, Tavanic, Tigeron, Flexid, Floracid, Hyleflox, Ecolevid, Eleflox | |
| Levofloxacin at Wikimedia Commons | |
Levofloxacin
is a drug, an antibacterial drug, part of the group of third generation fluoroquinolones. It is the L-enantiomer of ofloxacin with antibacterial activity twice as great as that of ofloxacin, which is a racemic mixture of L- and D-enantiomers[1].
pharmachologic effect
Fluoroquinolone is a broad-spectrum antimicrobial bactericidal agent. Blocks DNA gyrase (topoisomerase II) and topoisomerase IV, disrupts supercoiling and cross-linking of DNA breaks, suppresses DNA synthesis, causes profound morphological changes in the cytoplasm, cell wall and membranes of the bacterium.
Effective against Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Viridans group streptococci, Enterobacter cloacae, Enterobacter aerogenes, Enterobacter agglomerans, Enterobacter sakazakii, Escherichia coli, Haemophilus influenzae , Haemophilus parainfluenzae, Klebsiella pneumoniae, Klebsiella oxytoca, Legionella pneumoniae, Moraxella catarrhalis, Proteus mirabilis, Pseudomonas aeruginosa, Pseudomonas fluorescens, Chlamydia pneumoniae, Mycoplasma pneumoniae, Mycoplasma hominis, Acinetobacter anitratus, Acinetobacter baumannii, Acinetobacter calcoaceticus, Bordetella pertussis, Citrobacter diversus, Citrobacter freund ii, Morganella morganii, Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Serratia marcescens, Clostridium perfringens
.
Indications
Uncomplicated urinary tract infections caused by Escherichia coli
,
Klebsiella pneumoniae
, Proteus mirabilis (Gram-negative, sporogen-forming, facultative anaerobic bacteria) or Staphylococcus saprophyticus (a coagulase-negative species of staphylococci, a common cause of urinary tract infections).
Complicated urinary tract infections caused by Escherichia coli
,
Klebsiella pneumoniae
, Proteus mirabilis, Pseudomonas aeruginosa, Citrobacter diversus or Enterobacter cloacae. Prevention of urinary tract infections during transrectal prostate biopsy and transurethral surgical interventions. Exacerbation of chronic bronchitis caused by Haemophilus influenzae or Moraxella catarrhalis. Pulmonary tuberculosis (as part of complex therapy): extensive caseous-necrotic tissue lesions, a pronounced nonspecific component of inflammation, drug resistance of mycobacteria to rifampicin or poor tolerability of rifampicin.
Pharmacokinetics
When taken orally, it is quickly and almost completely absorbed (food intake has little effect on the speed and completeness of absorption). Bioavailability - 99%. TCmax - 1-2 hours; when taking 250 and 500 mg, Cmax is 2.8 and 5.2 μg/ml, respectively. Bonding with plasma proteins is 30-40%. Penetrates well into organs and tissues: lungs, bronchial mucosa, sputum, genitourinary organs, polymorphonuclear leukocytes, alveolar macrophages. In the liver, a small portion is oxidized and/or deacetylated. Renal clearance accounts for 70% of the total clearance. T
1/2 - 6-8 hours. Excreted from the body mainly by the kidneys through glomerular filtration and tubular secretion. Less than 5% of levofloxacin is excreted as metabolites. Unchanged in urine, 70% is excreted within 24 hours and 87% within 48 hours; 4% of the dose taken orally is found in feces within 72 hours. After intravenous infusion of 500 mg over 60 minutes, Cmax is 6.2 mcg/ml. With intravenous single and repeated administration, the apparent volume of distribution after administration of the same dose is 89-112 l, Cmax - 6.2 mcg/ml, T1/2 - 6.4 hours.
Release forms
On the pharmaceutical market, the antibiotic Levofloxacin is presented in several forms:
- Tablets 250 mg and 500 mg.
- Eye drops 0.5%.
- Solution for infusion 0.5%.
- Levofloxacin tablets are labeled “Levofloxacin 250” and “Levofloxacin 500” according to the dosage of the active substance, where the values of 250 and 500 determine the quantitative content of the antibiotic in the drug. The tablet is yellowish in color, round, biconvex in shape. The section clearly shows two layers. Levofloxacin in doses of 250 mg and 500 mg is presented in packages of 5 or 10 tablets.
- eye drops look like a clear, homogeneous, almost colorless liquid. The drops are presented in bottles of 5 ml or 10 ml, which have a special dropper-shaped cap for ease of use.
- infusion solution is presented in bottles (or ampoules) of 100 ml. One milliliter of the drug contains 5 mg of antibiotic. A whole bottle of solution for infusion (100 ml) contains 500 mg of active substance, which is used in the format of intravenous droppers.
- Store in a dry, dark and cool place (temperature no more than 25 °C).
- Keep away from children.
- tablets also contain a number of excipients:
Dosage regimen
Orally, before meals or between meals, without chewing, with a sufficient amount of liquid.
- For acute sinusitis - orally, 500 mg 1 time per day for 10-14 days, or 750 mg 1 time per day for 5 days;
- For infectious exacerbation of COPD - 500 mg 1 time per day for 7-10 days, or 750 mg 1 time per day for 5 days;
- For pneumonia - orally, 250-500 mg 1-2 times a day (0.5-1 g/day); intravenously - 500 mg 1-2 times a day, for 7-14 days, or 750 mg 1 time a day for 5 days.
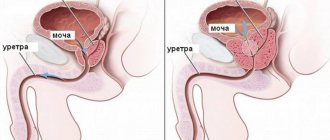
- For urinary tract infections - orally, 250 mg 1 time per day; intravenously, at the same dose, for 7-10 days. For prostatitis - orally, 500 mg 1 time per day for 28 days.
- For infections of the skin and soft tissues - orally, 250-500 mg 1-2 times a day; intravenously, 500 mg 2 times a day, for 7-14 days.
- For tuberculosis - orally, 500 mg 1-2 times a day for up to 3 months.
It is also used intravenously; after intravenous administration, after a few days it is possible to switch to oral administration at the same dose.
For kidney diseases, the dose is reduced in accordance with the degree of dysfunction: with creatinine clearance (CC) 20-50 ml/min - 125-250 mg 1-2 times a day, 10-19 ml/min - 125 mg 1 time in 12 -48 hours, less than 10 ml/min (including hemodialysis) - 125 mg after 24 or 48 hours.
Levofloxacin in the treatment of severe community-acquired pneumonia
Community-acquired pneumonia is one of the most common acute infectious diseases. According to foreign studies, the incidence of community-acquired pneumonia ranges from 1 to 11.5‰ in young and middle-aged people and reaches 25-44‰ in older age groups. In the United States, 3-4 million cases of community-acquired pneumonia are diagnosed annually, and more than 900,000 patients are hospitalized. Of those hospitalized, more than 60,000 people die directly from community-acquired pneumonia. Isolating patients with severe community-acquired pneumonia into a separate group is extremely important, given the high mortality rate, often the presence of severe background pathology, the etiology of the disease and special requirements for antibacterial therapy. If one of the following criteria is present, community-acquired pneumonia is considered severe (Table).
Table. Criteria for severe community-acquired pneumonia
| Clinical | Laboratory |
| Acute respiratory failure: • respiratory rate > 30 per minute • blood oxygen saturation < 90 mm Hg. Art. | Leukopenia (< 4 × 109/l) |
| Hypotension: • systolic blood pressure < 90 mm Hg. • diastolic blood pressure < 60 mm Hg. Art. | Hypoxemia: • SaO2 < 90% • PO2, < 60 mm Hg. Art. |
| Bi- or multilobar lesion | Hemoglobin < 100 g/l |
| Impaired consciousness | Hematocrit < 30% |
| Extrapulmonary focus of infection (meningitis, pericarditis, etc.) | Acute renal failure (blood creatinine > 176.7 µmol/l, urea nitrogen - 7 mmol/l) |
In severe community-acquired pneumonia, the prescription of antibacterial drugs should be urgent; a delay of 8 hours significantly worsens the prognosis. The drugs of choice are parenterally administered inhibitor-protected penicillins or fifth-generation cephalosporins in combination with macrolides. There is data from controlled clinical studies on the possibility of monotherapy with “respiratory” fluoroquinolones for severe community-acquired pneumonia. “Respiratory” fluoroquinolones have increased antipneumococcal activity and cover almost the entire spectrum of possible pathogens.
Under our supervision there were 23 patients with severe community-acquired pneumonia aged from 21 to 81 years (young and middle-aged people 85%); The average age of patients was 37.6 ± 4.65 years, the majority were women (about 61%). Bad habits: smoking and alcohol abuse were not denied by 58% of those surveyed. The duration of the disease before admission to the hospital ranged from 1 to 14 days (6.6 ± 1.8 on average). Due to the ineffectiveness of initial therapy with third-generation cephalosporins in combination with macrolides (in some patients, semisynthetic penicillins or I-II generation cephalosporins in combination with macrolides), Tavanic (levofloxacin) was prescribed at a dose of 500 mg intravenously 1 time per day. The duration of parenteral administration of Tavanik is 5-7 days; some patients were then prescribed oral administration at a dose of 500 mg per day for 3-5 days (if necessary, continued antibacterial therapy). The causative agent of community-acquired pneumonia was identified in 53% of patients: streptococcus pneumoniae, α-hemolytic streptococcus. Bacterioscopy revealed Gram+ and Gram− flora.
During treatment with Tavanik within 3 days, intoxication syndrome was eliminated in most patients; the temperature returned to normal after 3.16 ± 0.59 days; cough decreased on days 5-8; physical signs persisted longer - up to 10-14 days; resorption of pneumonic infiltration was noted after 16.7 ± 6.2 days (according to radiological data). Laboratory parameters underwent the following changes: leukocytosis with a shift to the left upon admission was noted in 83% of patients (leukocytes 13.95 ± 1.56 x 109/l, band neutrophils 15.62 ± 3.72%), on days 6-8 During hospital stay, leukocytosis with a shift to the left persisted in 31% of patients (leukocytes - 12.78 ± 0.58 x 109/l, band neutrophils - 13.4 ± 1.8%). In 69% of patients, the number of leukocytes returned to normal (6.55 ± 0.55 x 109/l) while maintaining a shift of the leukocyte formula to the left (band neutrophils 7.3 ± 1.21%). Normalization of the number of leukocytes in all patients occurred after the 10th day of illness (leukocytes 6.5 ± 0.41 x 109/l, band neutrophils 3.12 ± 0.38%). ESR remained elevated at the time of discharge of patients: 25.6 ± 2.3 mm/h (on admission 45.47 ± 3.83 mm/h, on day 6 - 44.06 ± 3.58 mm/h). It was possible to switch to oral administration of Tavanik no earlier than the 6th day of parenteral administration, taking into account the recommended criteria:
- regression of clinical manifestations of the disease;
- normal body temperature with two consecutive measurements with an 8-hour interval;
- decrease in the number of leukocytes in peripheral blood;
- absence of absorption disorders that would prevent the drug from being taken orally.
All patients experienced clinical and radiological recovery. No side effects of Tavanik were noted. For comparison, 13 case histories of patients with severe community-acquired pneumonia were analyzed, in whom initial therapy with third-generation cephalosporins or their combination with parenteral macrolides was effective. There were no statistically significant differences in the timing of relief of local and general symptoms, normalization of laboratory parameters and resorption of pulmonary infiltrate. Thus, if initial therapy with third-generation cephalosporins is ineffective, it is advisable to switch to parenteral administration of Tavanic as early as possible (48 hours). The advantage of Tavanik is its availability in both parenteral and oral forms, which determines the possibility of using it as part of step-down therapy.
Khrutskaya M. S., Konoshchuk T. Z., Pankratova Yu. Yu. Belarusian State Medical University, 10th City Clinical Hospital, Minsk.
(Published in the journal “Medical Panorama” No. 10, November 2004)
Side effects
From the digestive system:
nausea, vomiting, diarrhea (including blood), indigestion, loss of appetite, abdominal pain, pseudomembranous enterocolitis;
increased activity of “liver” transaminases, hyperbilirubinemia, hepatitis, dysbacteriosis. From the cardiovascular system:
decreased blood pressure, vascular collapse, tachycardia, prolongation of the QT interval.
Metabolism:
hypoglycemia (increased appetite, increased sweating, trembling).
From the nervous system:
headache, dizziness, weakness, drowsiness, insomnia, tremor, anxiety, paresthesia, fear, hallucinations, confusion, depression, movement disorders, epileptic seizures (in predisposed patients).
From the senses:
disturbances of vision, hearing, smell, taste and tactile sensitivity.
From the musculoskeletal system
: arthralgia, muscle weakness, myalgia, tendon rupture, tendinitis.
From the urinary system:
hypercreatininemia, interstitial nephritis, acute renal failure.
From the hematopoietic organs:
eosinophilia, hemolytic anemia, leukopenia, neutropenia, agranulocytosis, thrombocytopenia, pancytopenia, hemorrhages.
Allergic reactions:
photosensitivity, itching and redness of the skin, swelling of the skin and mucous membranes, urticaria, malignant exudative erythema (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome), bronchospasm, suffocation, anaphylactic shock, allergic pneumonitis, vasculitis.
Other:
asthenia, exacerbation of porphyria, rhabdomyolysis, persistent fever, development of superinfection.
Overdose
Symptoms:
nausea, erosive lesions of the mucous membranes of the gastrointestinal tract, prolongation of the QT interval, confusion, dizziness, convulsions.
Treatment:
symptomatic, dialysis is ineffective.
special instructions
After normalization of body temperature, it is recommended to continue treatment for at least 48-78 hours. The duration of intravenous infusion of 500 mg (100 ml of infusion solution) should be at least 60 minutes. During treatment, it is necessary to avoid solar and artificial UV irradiation to avoid damage to the skin (photosensitization). If signs of tendonitis or pseudomembranous colitis appear, levofloxacin is immediately discontinued. It should be borne in mind that in patients with a history of brain damage (stroke, severe trauma), seizures may develop; in patients with glucose-6-phosphate dehydrogenase deficiency, there is a risk of hemolysis. During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Levofloxacin
Patients predisposed to developing seizures
Like other quinolones, levofloxacin should be used with great caution in patients with a predisposition to seizures. These patients include patients with pre-existing central nervous system lesions such as stroke, severe traumatic brain injury, patients concomitantly taking drugs that lower the seizure threshold of the brain, such as fenbufen or other similar non-steroidal anti-inflammatory drugs or other drugs that lower seizure threshold, such as theophylline (see section "Interaction with other drugs").
Patients with glucose-6-phosphate dehydrogenase deficiency
Patients with latent or manifest glucose-6-phosphate dehydrogenase deficiency have an increased risk of hemolytic reactions when treated with quinolones, which should be taken into account when treated with levofloxacin.
Patients with impaired renal function
Since levofloxacin is excreted mainly through the kidneys, patients with impaired renal function require mandatory monitoring of renal function, as well as adjustment of the dosage regimen, see section “Dosage and Administration”.
When treating elderly patients, it should be borne in mind that patients in this group often have impaired renal function (see section “Dosage and Administration”).
QT prolongation
Very rare cases of QT prolongation have been reported in patients taking fluoroquinolones, including levofloxacin.
When using fluoroquinolones, including levofloxacin, caution should be exercised in patients with known risk factors for prolongation of the QT interval: in elderly patients, in female patients, in patients with uncorrected electrolyte disturbances (with hypokalemia, with hypomagnesemia), with congenital long QT syndrome , with heart disease (heart failure, myocardial infarction, bradycardia), while taking medications that can prolong the QT interval, such as class IA and III antiarrhythmics, tricyclic antidepressants, macrolides, antipsychotics.
Elderly and female patients may be more sensitive to drugs that prolong the QT interval.
Hypo and hyperglycemia (dysglycemia)
As with the use of other quinolones, cases of hyperglycemia and hypoglycemia have been observed when using levofloxacin, especially in patients with diabetes mellitus receiving concomitant oral hypoglycemic agents, such as glibenclamide or insulin preparations. Cases of hypoglycemic coma have been reported. In patients with diabetes mellitus, monitoring of blood glucose concentrations is required.
Severe neurological reactions
Patients with severe adverse reactions to other fluoroquinolones, such as severe neurological reactions, have an increased risk of experiencing similar adverse reactions when taking levofloxacin.
Psychotic reactions
With the use of quinolones, including levofloxacin, the development of psychotic reactions has been reported, which in rare cases progressed to the development of suicidal ideation and behavior disorders with self-harm (sometimes after administration of a single dose of levofloxacin). If such reactions develop, treatment with levofloxacin should be discontinued and appropriate treatment should be prescribed. The drug should be prescribed with caution to patients with psychosis or patients with a history of mental illness.
Peripheral neuropathy
Sensory and sensorimotor peripheral neuropathy, which may have a rapid onset, has been reported in patients taking fluoroquinolones, including levofloxacin. If the patient develops symptoms of neuropathy, levofloxacin should be discontinued. This minimizes the possible risk of developing irreversible changes.
Exacerbation of pseudoparalytic myasthenia gravis (myasthenia gravis)
Fluoroquinolones, including levofloxacin, have neuromuscular blocking activity and may increase muscle weakness in patients with myasthenia gravis. Post-marketing adverse reactions, including pulmonary failure requiring mechanical ventilation and death, have been associated with the use of fluoroquinolones in patients with myasthenia gravis. The use of levofloxacin in a patient with an established diagnosis of pseudoparalytic myasthenia gravis is not recommended.
Hypersensitivity reactions
Levofloxacin may cause serious, potentially fatal hypersensitivity reactions (angioedema, anaphylactic shock) (see section "Side effects") Patients should immediately stop taking the drug and consult a doctor.
Severe bullous reactions
Cases of severe bullous skin reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis have been observed while taking levofloxacin (see section "Side effects"). In case of development of any reactions from the skin or mucous membranes, the patient should immediately consult a doctor and not continue treatment until his consultation.
Disorders of the liver and biliary tract
Cases of hepatic necrosis, including the development of fatal liver failure, have been reported with the use of levofloxacin, mainly in patients with severe underlying diseases, such as sepsis (see section "Side effects"). Patients should be warned to stop treatment and seek immediate medical attention if signs and symptoms of liver damage occur, such as anorexia, jaundice, dark urine, itching and abdominal pain.
Interaction
Increases T1/2 of cyclosporine. The effect is reduced by drugs that inhibit intestinal motility, sucralfate, Al3+ and Mg2+-containing antacid drugs and Fe salts (a break of at least 2 hours is required between doses). NSAIDs, theophylline increase convulsive readiness, corticosteroids increase the risk of tendon rupture. Cimetidine and drugs that block tubular secretion slow down excretion. The solution for intravenous administration is compatible with 0.9% NaCl solution, 5% dextrose solution, 2.5% Ringer's solution with dextrose, combined solutions for parenteral nutrition (amino acids, carbohydrates, electrolytes). Do not mix with heparin and alkaline solutions.
Drug interactions
There are reports of a pronounced decrease in the cerebral seizure threshold when using quinolones in combination with substances that can, in turn, reduce the cerebral seizure threshold. This equally applies to the simultaneous use of quinolones with theophylline.
A significant weakening of the effect of levofloxacin is observed when it is used in combination with sucralfate, iron salts, aluminum or magnesium-containing antacids. In this regard, levofloxacin should be taken no earlier than 2 hours before or 2 hours after taking these drugs.
Cimetidine and probenecid slightly slow down the elimination of levofloxacin. This interaction has virtually no clinical significance, however, when using such combinations, therapy should be carried out with caution, especially in patients with limited renal function.
It should be taken into account that glucocorticosteroids taken simultaneously with levofloxacin increase the risk of tendon rupture.
No interaction of levofloxacin with calcium carbonate has been identified; When used with vitamin K antagonists, monitoring of the blood coagulation system is necessary.
Levofloxacin increases the half-life of cyclosporine.